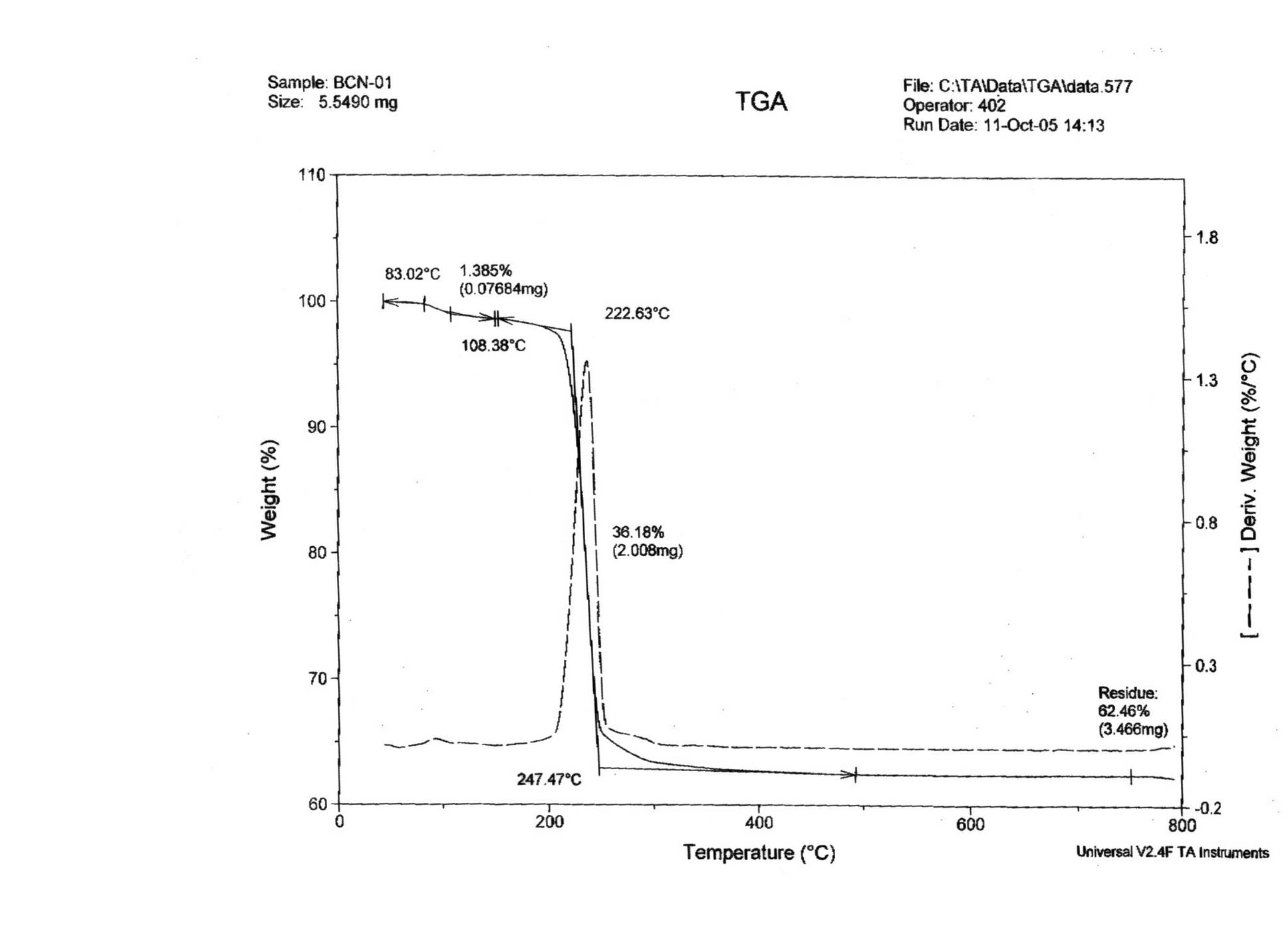
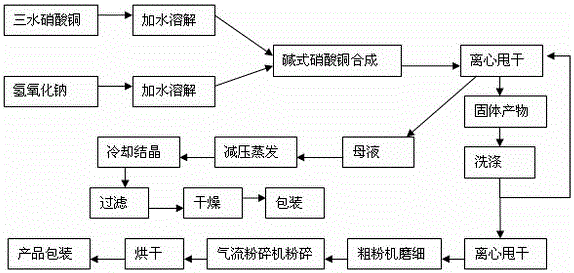
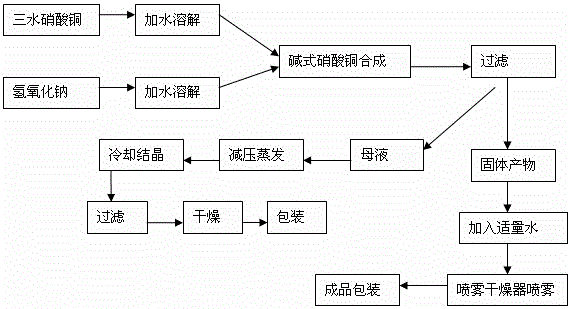
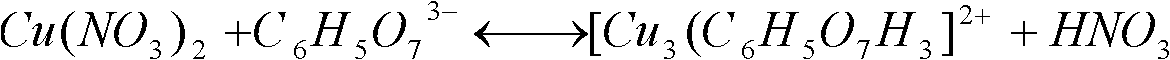Patents
Literature
39results about "Copper nitrates" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Direct synthesis of copper carbonate
ActiveUS20070269362A1High purityEasy to convertGroup 1/11 element organic compoundsCopper ammonia complexesOxygenCopper carbonate
A basic copper salt selected from basic copper carbonate, basic copper sulfate, basic copper acetate, basic copper citrate, and basic copper nitrate is manufactured by contacting copper metal with an aqueous solution having ammonia; an acid selected from carbonic acid, sulfuric acid, acetic acid, nitric acid, or citric acid; and oxygen, under conditions where the copper metal is converted to the basic copper salt; and then recovering the basic copper salt. The most economical embodiment is where the ammonia is present in the aqueous solution is in an amount between about 6.7 g / l and about 15 g / l calculated as NH3, and the pH of the composition is between 8 and 10, and the temperature of the composition is between 25° C. and 100° C. The method is particularly useful if the basic copper salt is basic copper carbonate. The basic copper carbonate produced has the formula: (CuCO3)x(Cu(OH2)y, where y is 1 and x is between 0.1 to less than 1; or where y is 1 and x is 1, or where y is 1 and x is between 0.5 to less than about 0.95, or where y is 1 and x greater than 1.
Owner:KOPPERS PERFORMANCE CHEM
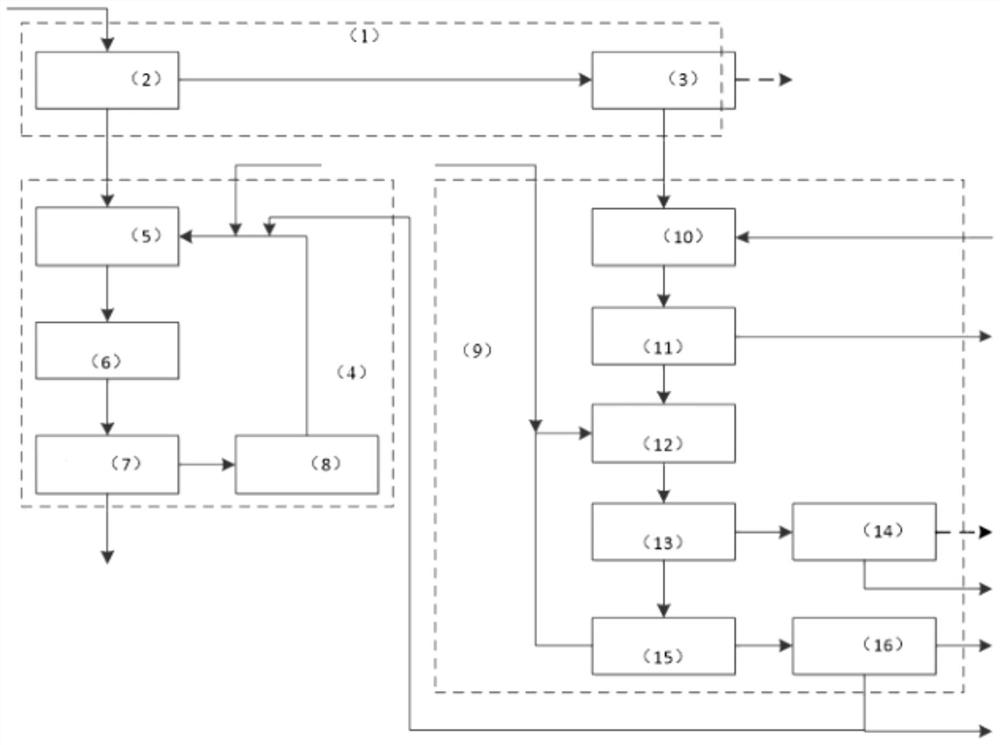
Comprehensive utilization method of waste liquor in production of basic cupric carbonate
ActiveCN103449501AAchieve reuseRealize recycling of resourcesCopper chloridesCopper nitratesSulfate radicalsIndustrial waste water
The invention discloses a comprehensive utilization method of waste liquor in the production of basic cupric carbonate and relates to the field of treatment methods of industrial wastewater. The invention aims at providing a comprehensive utilization method of waste liquor in the production of basic cupric carbonate, and in particular relates to a method for reusing sodium bicarbonate in the waste liquor. The waste liquor in the production of the basic cupric carbonate is the waste liquor generated after the basic cupric carbonate is produced by a reaction between an acidic copper chloride solution or an acidic copper sulfate solution and a sodium carbonate solution. The comprehensive utilization method of the waste liquor in the production of the basic cupric carbonate mainly comprises the following steps: adding a little distilled water into a reaction kettle as a base solution; when the reaction temperature rises to 35-90 DEG C, starting a stirring device, and adding the waste liquor containing sulfate radicals or chlorine and the acidic copper solution into the reaction kettle for reaction, wherein the pH value during the addition of the solutions is controlled to be 3-6; and filtering, washing, drying and sieving reaction products to obtain the basic copper salt. The method disclosed by the invention is simple to operate, easy to control reaction conditions, and widely applicable to the recycling and reusing of the waste liquor in the production of basic cupric carbonate.
Owner:SHENZHEN SHENTOU ENVIRONMENT TECH CO LTD
Direct synthesis of copper carbonate
ActiveUS7411080B2High purityEasy to convertNitrogen-metal/silicon/boron binary compoundsCopper organic compoundsCopper nitrateOxygen
A basic copper salt selected from basic copper carbonate, basic copper sulfate, basic copper acetate, basic copper citrate, and basic copper nitrate is manufactured by contacting copper metal with an aqueous solution having ammonia; an acid selected from carbonic acid, sulfuric acid, acetic acid, nitric acid, or citric acid; and oxygen, under conditions where the copper metal is converted to the basic copper salt; and then recovering the basic copper salt. The most economical embodiment is where the ammonia is present in the aqueous solution is in an amount between about 6.7 g / l and about 15 g / l calculated as NH3, and the pH of the composition is between 8 and 10, and the temperature of the composition is between 25° C. and 100° C. The method is particularly useful if the basic copper salt is basic copper carbonate. The basic copper carbonate produced has the formula: (CuCO3)x(Cu(OH2)y, where y is 1 and x is between 0.1 to less than 1; or where y is 1 and x is 1, or where y is 1 and x is between 0.5 to less than about 0.95, or where y is 1 and x greater than 1.
Owner:KOPPERS PERFORMANCE CHEM
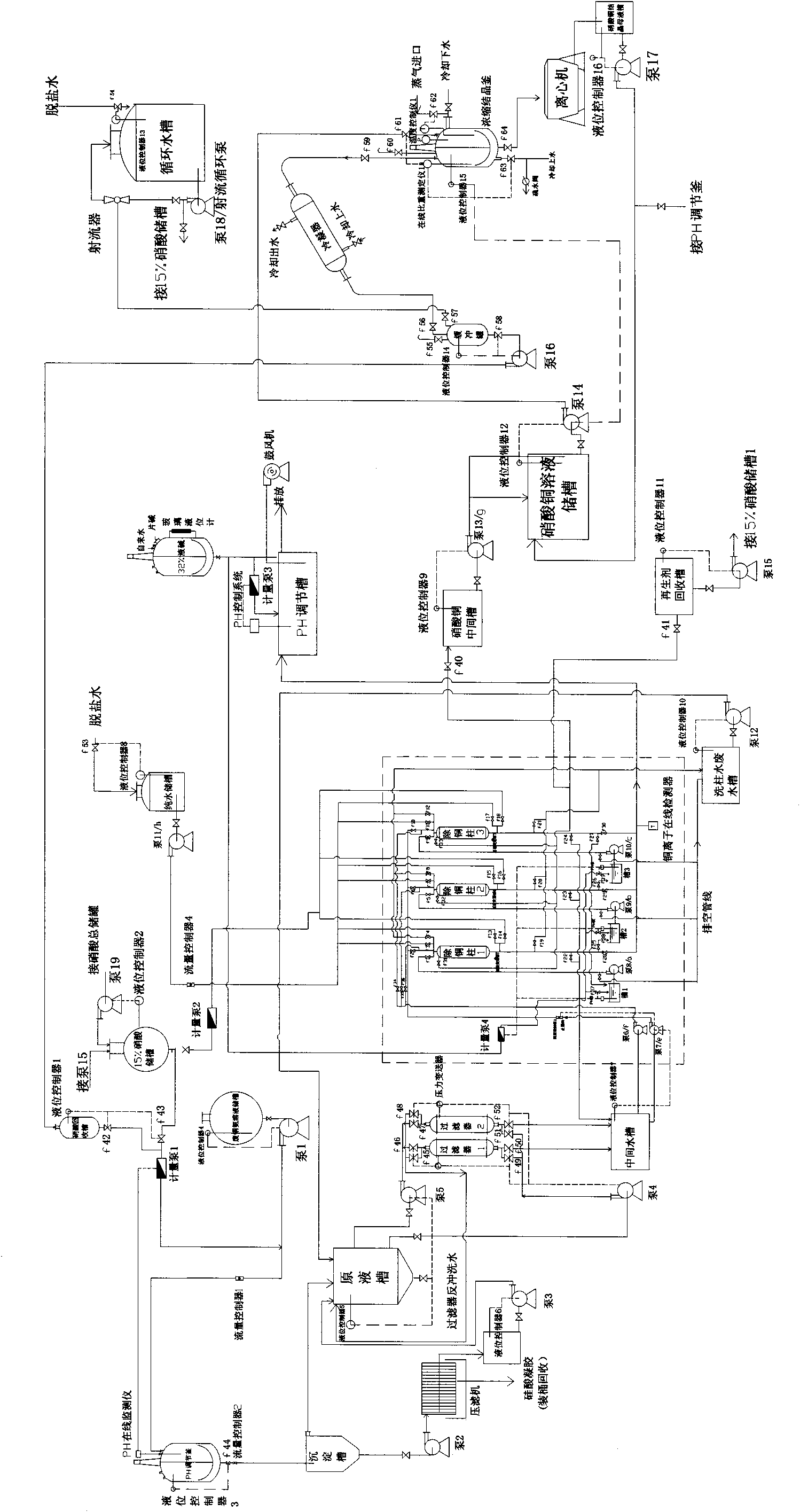
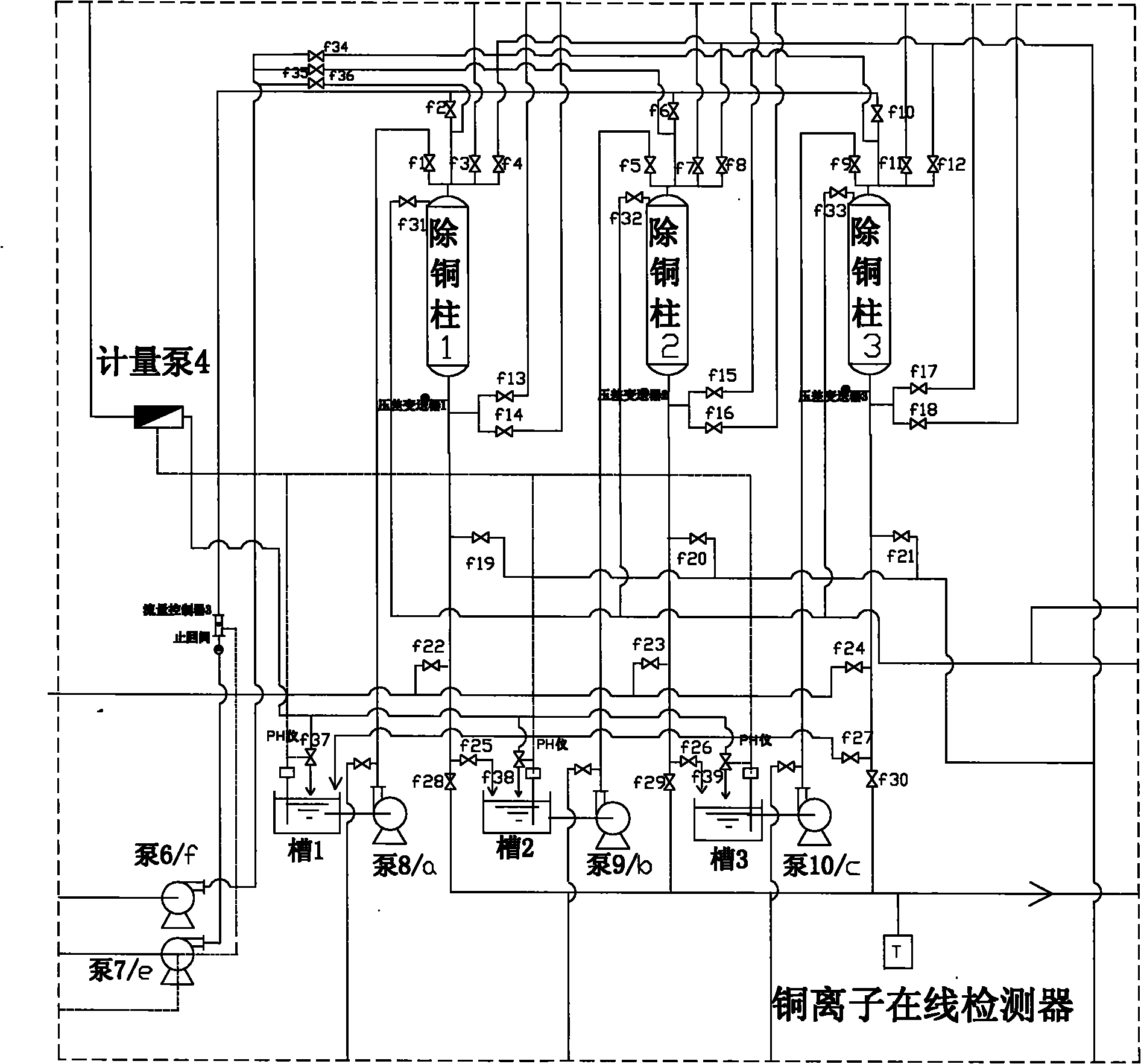
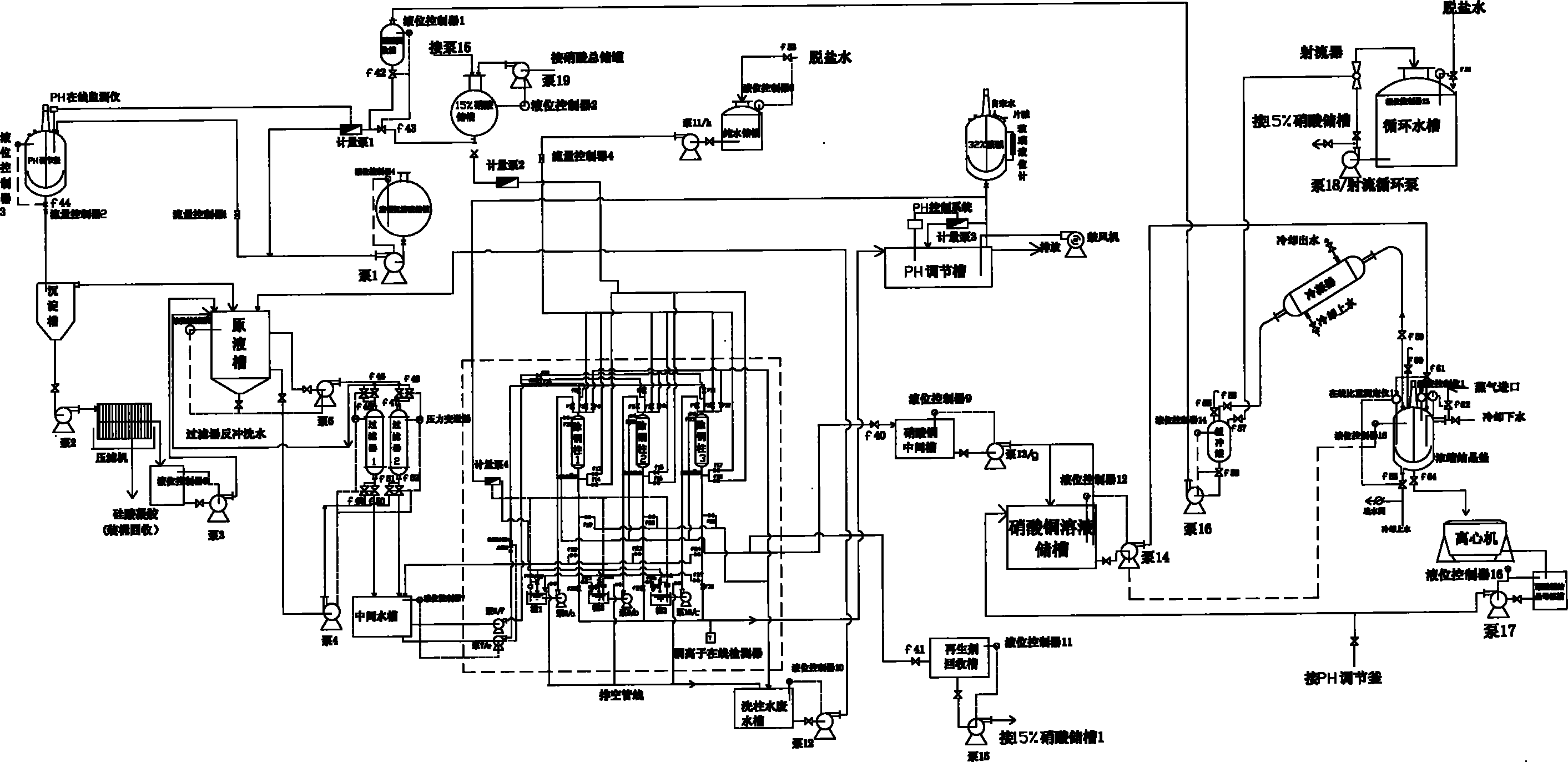
Technology for extracting copper from copper ammonia wastewater through ion exchange method
InactiveCN101863568AGuaranteed emission standardsHigh purityWater/sewage treatment by ion-exchangeMultistage water/sewage treatmentAutomatic controlIon exchange
The invention discloses a technology for extracting copper from copper ammonia wastewater through an ion exchange method. The technology comprises the following steps: firstly regulating the PH value of the wastewater to 2-3 to enable copper in the wastewater to exist in form of ions, simultaneously settling silicon ions and ferric ions in the wastewater, using a copper resin column to adsorb copper ions in the wastewater after impurities in the wastewater are removed, using nitric acid with weight in weight (w / w) of 8-15 percent for regeneration, and centrifugally separating the regenerated concentrated solution to obtain copper nitrate trihydrate. The technology for extracting copper from copper ammonia wastewater through the ion exchange method can be used for directly extracting copper ions from the wastewater. Since the system adopts online detection and automatic control, the wastewater emission is effectively ensured to satisfy the standard. The content of copper in the wastewater treated by using the technology of the invention is less than 0.5mg / l, the metal recovery rate can reach 99.5 percent and the purity of the recovered copper can reach 99 percent. Compared with a traditional ion exchange system, the invention has the advantages that the operation of the technology is simple, the operation cost is low, the high-purity copper nitrate can be obtained and the steam consumption is decreased by more than one time.
Owner:JIANGDU HAIYANG CHEM
Basic metal nitrate, process for producing the same and gas generating agent composition
InactiveUS20070119530A1Enhanced interactionDecrease in decomposition temperatureNitrogen-metal/silicon/boron binary compoundsExplosivesGuanidine derivativesSlag
Owner:ZHOU XINGXI +5
Clean method for preparing electronic grade high-purity copper nitrate solution
The invention discloses a clean method for preparing an electronic grade high-purity copper nitrate solution. The method comprises the following steps: after injecting dilute nitric acid into a reactor which resists the erosion of nitric acid, putting a superfluous electrolytic metallic copper plate into the reactor, stirring after dissolution reaction began, adding thicker hydrogen peroxide and thicker nitric acid, and carrying out solid-liquid separation after finishing the reaction to obtain the copper nitrate solution. The method has simple operation and no nitrogen oxide pollution.
Owner:广东光华化学厂有限公司
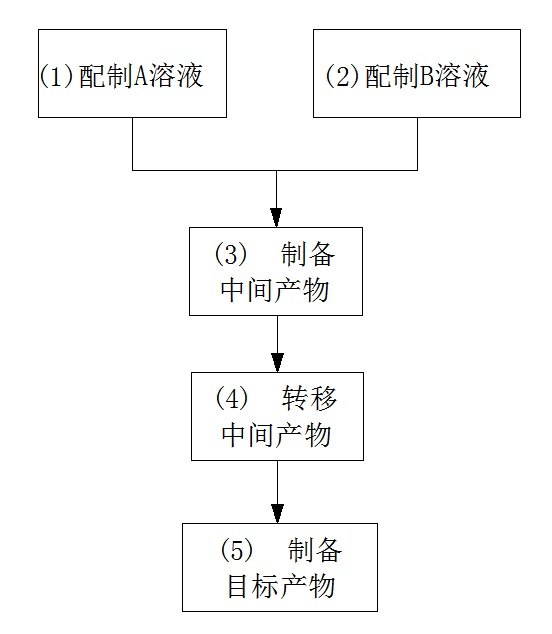
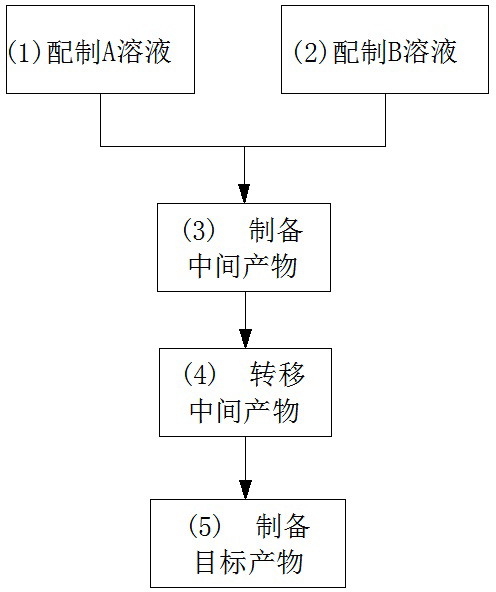
Preparation method for basic copper nitrate
The invention discloses a preparation method for basic copper nitrate, and the method is characterized by comprising the following steps of: preparing a solution A containing Cu<2+> and NO3<-> in a first reactor; preparing a solution B containing NH4<+> and OH<-> in a second reactor; enabling the solution A and the solution B to flow into a third reactor simultaneously, reacting in a water phase, adjusting the flow rates of the solution A and the solution B to cause the pH value of the reaction solution in the third reactor to be 3-10, and then preparing an intermediate product; transferring the intermediate product in a fourth reactor, washing until a washing solution is neutral, and then performing primary dewatering treatment to prepare a primary product; performing a series of subsequent treatments such as dehydration on the primary product in a fifth reactor, and then preparing a target product, namely basic copper nitrate. The preparation method for basic copper nitrate is increased in production efficiency by using the raw materials which are low in price and easy to get, simple equipment and simple technological processes, good and stable in product quality, and good in social and economic benefits.
Owner:苏州长湖纳米科技有限公司
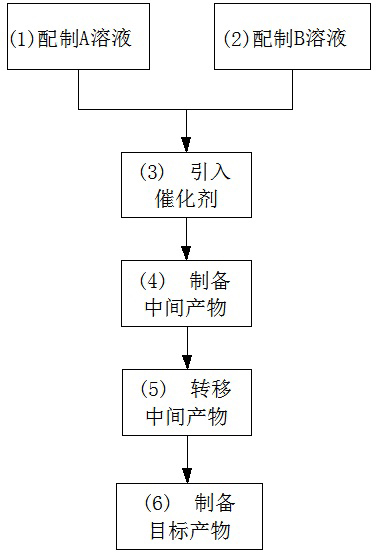
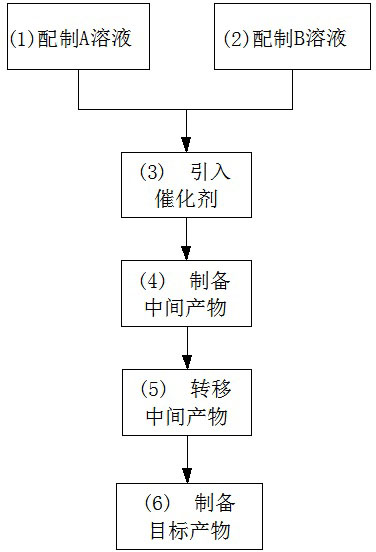
Preparation method for basic copper nitrate
InactiveCN102633290AReduce manufacturing costImprove product qualityCopper nitratesCopper nitratePhysical chemistry
The invention discloses a preparation method for basic copper nitrate, and the method comprises the following steps of: preparing a solution A containing Cu<2+> and NO3<->; preparing a solution B containing OH<->; introducing a citrate ion catalyst; preparing an intermediate product; transferring the intermediate product; and preparing a target product, namely basic copper nitrate. The preparation method for basic copper nitrate has the following advantages that: the raw materials which are low in price and easy to get are used, and the catalyst is introduced during the reaction process, thus increasing the production efficiency; simultaneously, production equipment is simplified, and technological processes are reduced, thus being more beneficial to industrial production and operation, and then lowering the production cost of the basic copper nitrate. Besides, the basic copper nitrate product prepared by the method disclosed by the invention is good and stable in quality.
Owner:廖勇志
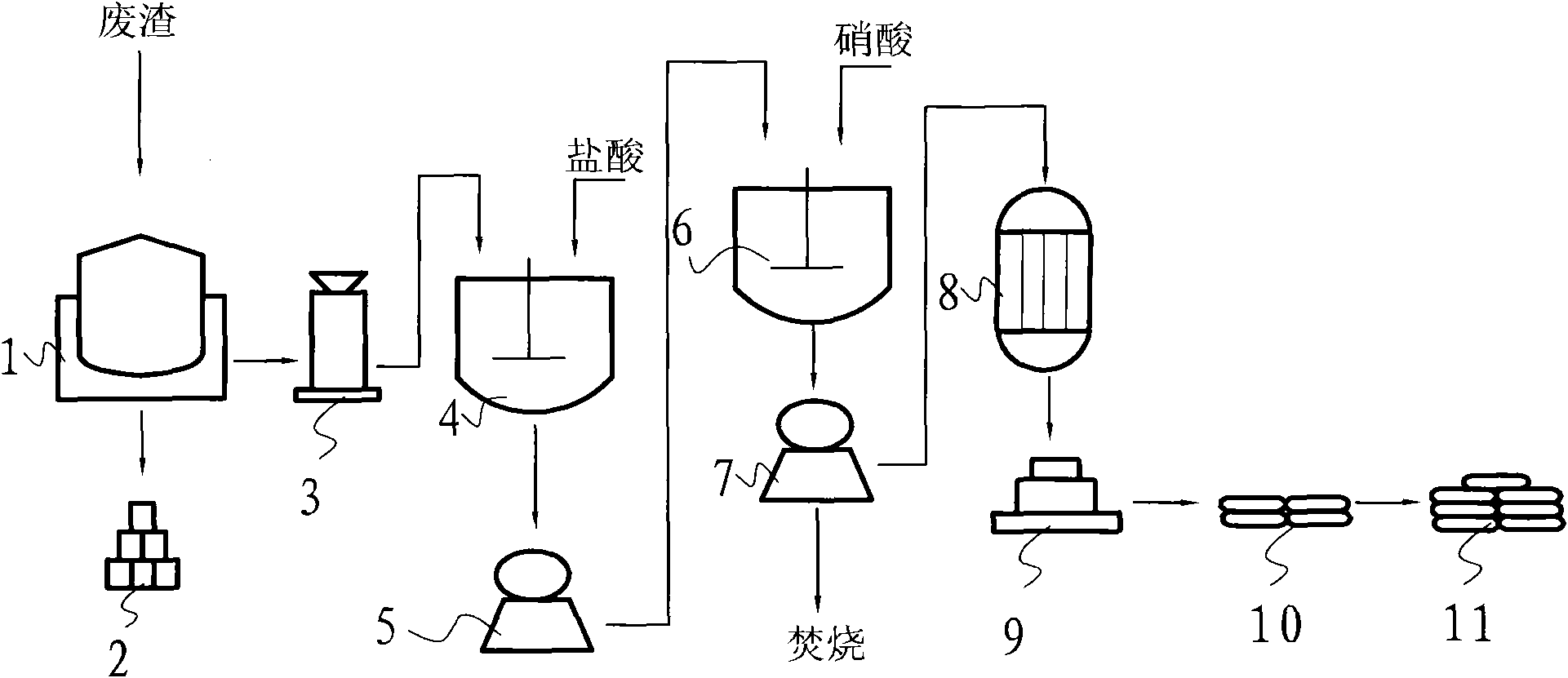
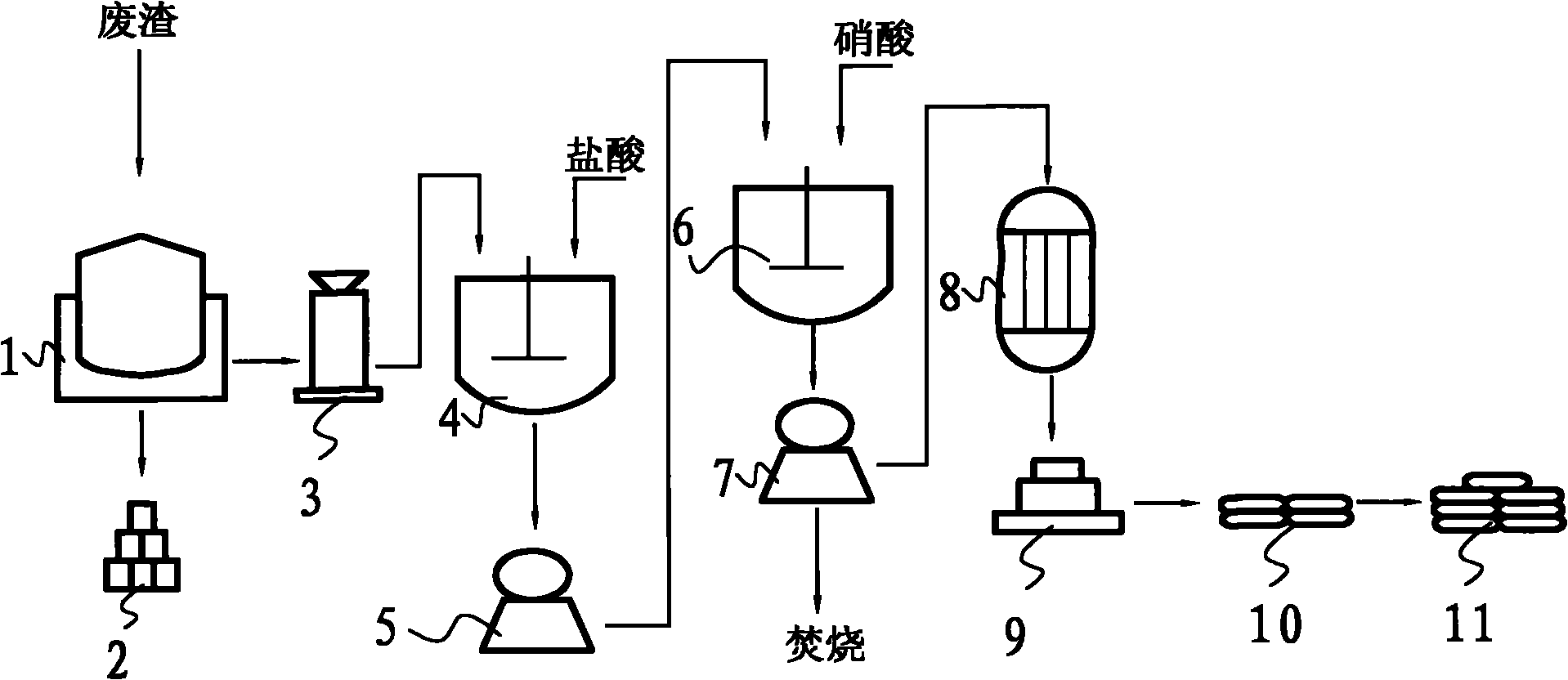
Recycling method of electroplating wastewater containing copper nitrate
ActiveCN103408164AShort processWide concentration rangeWater contaminantsWaste water treatment from metallurgical processCalcium hydroxideCopper nitrate
The invention provides a recycling method of electroplating wastewater containing copper nitrate and relates to the field of treatment methods of electroplating wastewater, aiming at providing a treatment method of electroplating wastewater containing copper nitrate, which is low in treatment cost, simple to operate, wide in application range, diversified in recycled products and relatively high in added value of products. The method mainly comprises the following steps: adding a small amount of distilled water into a reaction kettle to serve as base solution, starting a stirring device when the reaction temperature rises to 35-90 DEG C, adding the pre-treated electroplating wastewater containing copper nitrate and calcium oxide, calcium carbonate or calcium hydroxide slurry into the reaction kettle to react, filtering, washing, drying and sieving the reaction product to obtain an alkali type copper nitrate product, and carrying out concentration evaporating and crystallizing the filtrate to prepare a calcium nitrate product. The treatment method provided by the invention has the advantages that process flow is short, operation conditions are easily controlled, the method has safe and environment-friendly function, applicable treatment concentration range of electroplating wastewater containing copper nitrate is wide, and the method is particularly suitable for recycling copper in copper nitrate wastewater for small and medium enterprises.
Owner:SHENZHEN SHENTOU ENVIRONMENT TECH CO LTD
Sustainable recovery of metal compounds
InactiveUS20110274598A1Low costHigh purityCobalt ammonia complexesCopper oxides/halidesMetal nitrateManganese
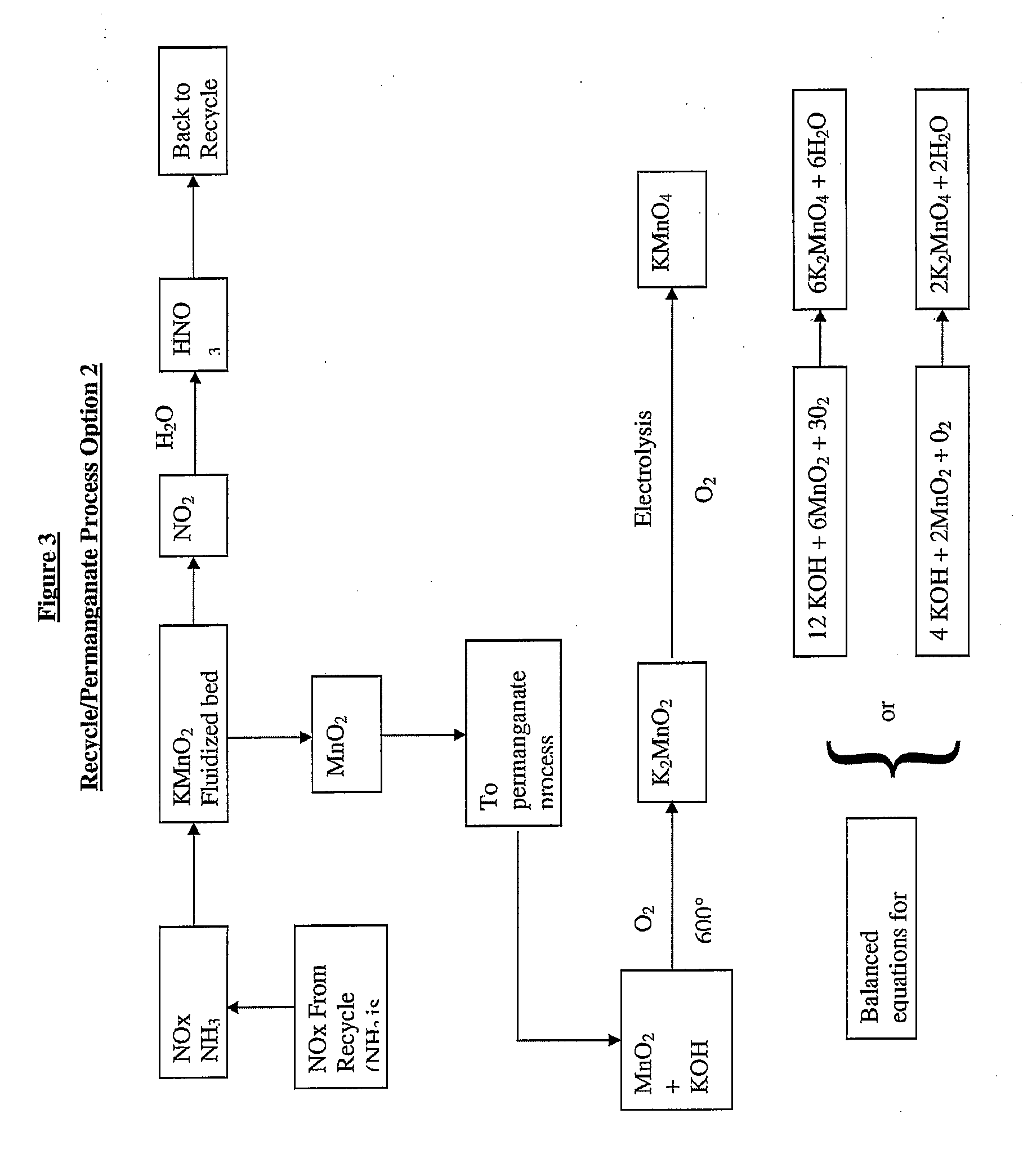
Disclosed is a process for removing metals from waste, particularly electronic waste (or “e-waste”). The process generally includes the steps of dissolving at least some of the metals from the waste with nitric acid reagent and then causing at least some of the metals to precipitate as metal oxides and / or metal nitrates. NOx gases produced as by-product by the nitric acid dissolution of metallic components in the electronic waste are reused, in particular for generating permanganate when one of the metallic components comprises manganese.
Owner:AKRIDGE JAMES R
Preparation of cupric nitrate solution
InactiveCN101481133AReduce consumptionAvoid it happening againCopper nitratesChemical reactionReaction temperature
The invention provides a method for preparing a cupric nitrate solution, which comprises the following steps: putting metal copper inside a pressure-proof and nitric acid corrosion-resistant container, and introducing industrial pure oxygen to perform the dissolving reaction of metal copper at a reaction temperature of between 20 100 DEG C and 100 DEG C with a reaction pressure between 0.05 MPa and 0.5 MPa. The nitric acid for reaction is between 1 mol / L and 14 mol / L. The amount of copper for reaction is larger than the amount calculated by the chemical equation and the reaction lasts for 1 to 10 hours.
Owner:SICHUAN NORMAL UNIVERSITY
Method for producing basic metal nitrate
A process in which a basic metal nitrate is obtained in a high yield is provided. A process for producing a basic metal nitrate, which comprises adding an aqueous solution of a metal nitrate or an aqueous solution of a mixture of a metal nitrate and a water-soluble additive and an alkali solution to a reaction vessel in which a reaction solvent whose pH at 20° C. is adjusted to 6 or less is present, and conducting the reaction with stirring.
Owner:DAICEL CHEM IND LTD
Preparation method of atmospheric pressure plasma of basic copper nitrate
Owner:中消天盾等离子科技(大连)有限公司
Preparation method of superfine basic copper nitrate for gas generator
A preparation method of superfine basic copper nitrate for a gas generator comprises the steps: 1, preparing aqueous solutions of cupric nitrate trihydrate and sodium hydroxide; 2, adding the sodium hydroxide aqueous solution and an additive into a reaction kettle, uniformly adding the copper nitrate aqueous solution into the reaction kettle within 0.5 h, controlling the pH value of the reaction solution of 7.5-9.0, and with the temperature of 60-90 DEG C, carrying out a reaction for 0.5-3 h; 3, carrying out filtration of a precipitation, retaining a mother liquor, adding water into the precipitation, pumping the suspension of the precipitation into a spray dryer for spray drying, and then packaging; and 4, pumping the mother liquor into a vacuum evaporator, carrying out reduced pressure evaporation, carrying out cooling crystallization, filtering, and drying the crystals for package. The crude product which can prevent aggregation or can prolong the time required to aggregate is obtained, and the steps of centrifugally spin-drying and multiple-time washing, levigating by a coarse powder machine and further crushing by an airflow crusher are omitted, so that the time is saved, the equipment investment is saved, moreover, the energy consumption and environmental pollutions are also reduced, the cost of the product is reduced, and the product can reach a same granularity level by a traditional process.
Owner:襄阳汉伟化工科技有限公司
A kind of preparation method of ultrafine basic copper nitrate for gas generator
The invention discloses a preparation method of ultrafine basic copper nitrate for a gas generator. Including ① configuring the aqueous solution of copper nitrate trihydrate and sodium hydroxide; ② adding sodium hydroxide and additives, adding the aqueous solution of copper nitrate evenly into the reaction kettle within 0.5h, controlling the pH value of the reaction solution to 7.5-9.0, and the temperature at 60- 90°C, react for 0.5-3h; ③Filtrate the precipitate, keep the mother liquor, add water to the precipitate, pump the precipitate suspension into a spray dryer for spray drying, and then pack; ④Pump the mother liquor into a vacuum evaporator and evaporate under reduced pressure , cooling and crystallization, the crystallization is filtered, dried and packaged. The present invention obtains a coarse product that can prevent agglomeration or prolong the time required for agglomeration as much as possible, and saves the steps of centrifugal drying for multiple times of washing, coarse powder machine grinding, and jet mill for further crushing, which not only saves time and equipment The investment also reduces energy consumption and environmental pollution, reduces product costs, and the product can reach the same granularity level as the traditional process.
Owner:襄阳汉伟化工科技有限公司
Basic metal nitrate, process for producing the same and gas generating agent composition
InactiveUS20090101250A1Improve processingIncrease burn rateNitrogen-metal/silicon/boron binary compoundsExplosivesGuanidine derivativesTetrazole
Provided is a basic metal nitrate suitable as an oxidizing agent for a gas generating agent, which is a basic metal nitrate having a good thermal stability and meeting at least one requirement of the following (a) to (d):(a) a particle diameter of 0.5 to 40 μm; (b) a degree of crystallinity having 0.4 deg or less of a half band width of the peak in the X-ray analysis; (c) an initiation temperature of weight loss being 220° C. or higher according to TG-DTA analysis; and (d) an impurity content of 1,000 ppm or less based on Na atom. Further provided is a gas generating composition which has a low toxicity, a high burning rate and a low combustion temperature and which is used in a gas generator for an air bag. The gas generating composition comprises (a) tetrazole derivatives, guanidine derivatives or a mixture thereof, (b) a basic metal nitrate and (c) a binder and / or a slag-forming agent.
Owner:ZHOU XINGXI +5
Method for preparing copper nitrate from nitric acid stripping liquid
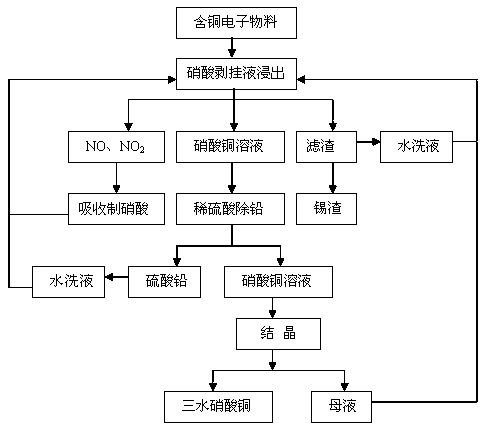
InactiveCN107935021ARealize recycling of resourcesLow priceCopper nitratesProcess efficiency improvementSlagElectronic materials
The invention relates to a method for resource treatment by using a nitric acid stripping liquid, and in particular relates to a method for preparing copper nitrate from the nitric acid stripping liquid. The method for preparing the copper nitrate by using the nitric acid stripping liquid is characterized by comprising the following steps: (1) performing leaching: using the nitric acid stripping liquid as a leaching solvent, adding a copper-containing electronic material for leaching, and after a leaching reaction is finished, performing solid-liquid separation to obtain a leaching liquid andtin slag; (2) removing lead: adding dilute sulfuric acid to the leaching liquid for removing the lead, and performing solid-liquid separation to obtain a copper nitrate solution and a lead sulfate precipitate; and (3) performing crystallization: performing crystallization on the copper nitrate solution to obtain industrial-grade copper nitrate. The method provided by the invention adopts the nitric acid stripping liquid and the copper-containing electronic material as raw materials to realize the resource recovery of tin and the lead, so as to obtain the tin slag and industrial-grade lead sulfate, and then crystallization purification is performed on the copper nitrate solution to obtain the industrial-grade copper nitrate. The method provided by the invention is simple in process, saves energy, protects environments, has high added value, and achieves the effects of turning waste into treasure and promoting resource benefits.
Owner:长沙湘朴科技有限公司
Preparation method for spectrograde copper nitrate
The present invention relates to the field of preparation methods for inorganic compounds, in particular to a preparation method for spectrograde copper nitrate. The invention is characterized by reacting nitric acid with electrolytic copper; introducing hydrogen sulfide gas to produce sulfide precipitates so as to remove metal cations; recrystallizing the copper nitrate to obtain spectrograde copper nitrate. The spectrograde copper nitrate prepared by the present invention has high purity and low impurity content, and the preparation process has easy steps and high benefits.
Owner:TIANJIN CHEM REAGENT RES INST
Technology for extracting copper from copper ammonia wastewater through ion exchange method
InactiveCN101863568BGuaranteed emission standardsHigh purityWater/sewage treatment by ion-exchangeMultistage water/sewage treatmentAutomatic controlIon exchange
The invention discloses a technology for extracting copper from copper ammonia wastewater through an ion exchange method. The technology comprises the following steps: firstly regulating the pH value of the wastewater to 2-3 to enable copper in the wastewater to exist in form of ions, simultaneously settling silicon ions and ferric ions in the wastewater, using a copper resin column to adsorb copper ions in the wastewater after impurities in the wastewater are removed, using nitric acid with weight in weight (w / w) of 8-15 percent for regeneration, and centrifugally separating the regenerated concentrated solution to obtain copper nitrate trihydrate. The technology for extracting copper from copper ammonia wastewater through the ion exchange method can be used for directly extracting copper ions from the wastewater. Since the system adopts online detection and automatic control, the wastewater emission is effectively ensured to satisfy the standard. The content of copper in the wastewater treated by using the technology of the invention is less than 0.5mg / l, the metal recovery rate can reach 99.5 percent and the purity of the recovered copper can reach 99 percent. Compared with a traditional ion exchange system, the invention has the advantages that the operation of the technology is simple, the operation cost is low, the high-purity copper nitrate can be obtained and the steam consumption is decreased by more than one time.
Owner:JIANGDU HAIYANG CHEM
Preparation method of basic copper nitrate
Owner:南京逸柔蒂雯新材料科技有限公司
Method for preparing alkali type copper nitrate ultrafine powder with microwave hydrothermal method
The invention discloses a method for preparing alkali type copper nitrate ultrafine powder with a microwave hydrothermal method. The method comprises the steps that a copper nitrate water solution is prepared and injected into a reaction kettle with a polytetrafluoroethylene lining, the reaction kettle is placed into a microwave hydrothermal device to be heated, a sodium hydroxide or potassium hydroxide water solution is dropwise added to adjust the reaction liquid pH, the precipitate is immersed with a dilute nitric acid water solution and stirred and washed, a solid is placed into a dryer to be dried to obtain the alkali type copper nitrate ultrafine powder, and the production cost can be reduced with the method.
Owner:FOSHAN XILONG CHEM CO LTD
Waste acid recovery system and method
PendingCN111675404ANo pollution in the processAvoid consumptionPreparation from carboxylic acid saltsCalcium/strontium/barium fluoridesAcetic acidO-Phosphoric Acid
The invention discloses a waste acid recovery system, which comprises a waste acid pre-rectification device (1), the waste acid pre-rectification device (1) is connected with a phosphoric acid pre-rectification device (4); and the phosphoric acid pre-rectification device (4) is communicated with a post-treatment device (9). Resourceful, harmless and cyclic comprehensive utilization of waste acid is realized; poisonous and harmful substances in the waste acid are recycled, valuable components are recovered and made into phosphoric acid, acetic acid and copper nitrate; phosphoric acid, acetic acid and copper nitrate can be directly sold as chemical raw materials, returned to the market again and utilized; generated calcium fluoride and calcium sulfate are treated as general waste, and generated waste gas is treated by an environment protection device and then discharged after reaching the standards.
Owner:绵阳市鑫科源环保科技有限公司
Method for producing cadmium nitrate and copper nitrate in preparation of cast iron ingots by arc process mixed iron slag
InactiveCN101967564AReduce pollutionSimple processProcess efficiency improvementCopper nitratesElectric arc furnaceSlag
The invention belongs to the technical field of comprehensive use of waste lead slag from manufacturing of inorganic chemical lithopone, in particular to a method for producing cadmium nitrate and copper nitrate in preparation of cast iron ingots by arc process mixed iron slag. The invention adopts a main technical scheme that: an arc furnace technique is used to extract the heavy metal from the lithopone waste slag; the waste slag is subjected high-temperature treatment, acidic treatment and distillation treatment; and thus, monomer iron, copper and cadmium and valuable compounds thereof are prepared. The method can remove heavy metal slag completely and reduces the pollution of the heavy metal to environment. The method can prepare valuable products and can create great social and economic benefit.
Owner:王嘉兴
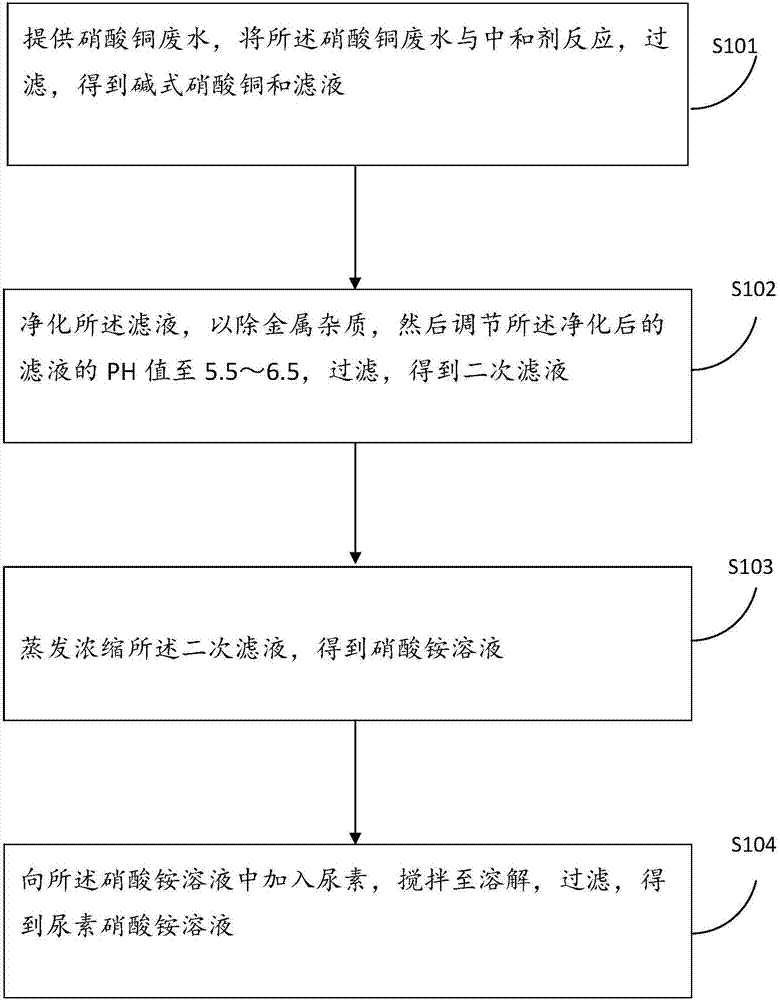
Copper nitrate-containing wastewater recovery method, and urea-ammonium nitrate liquid fertilizer preparation method
ActiveCN107266129ARealize resourcesSimple production processAmmonium nitratesAmmonium nitrate fertilisersRecovery methodMetal impurities
The invention provides a copper nitrate-containing wastewater recovery method. The method comprises the following steps: providing copper nitrate wastewater, reacting the copper nitrate wastewater with a neutralizer, and filtering the neutralized wastewater to obtain copper nitrate basic and a filtrate; purifying the filtrate to remove metal impurities, adjusting the pH value of the purified filtrate to 5.5-6.5, and filtering the filtrate to obtain a secondary filtrate; carrying out evaporative concentration on the secondary filtrate to obtain an ammonium nitrate solution; and adding urea to the ammonium nitrate solution, stirring the urea and the ammonium nitrate solution until the urea is dissolved, and filtering the obtained solution to obtain a urea-ammonium nitrate solution. The recovery method has the advantages of simple production process, low cost, effective realization of complete recycling of copper and nitrate ions in the copper nitrate wastewater, and no secondary pollution. The invention also provides a preparation method of a urea-ammonium nitrate liquid fertilizer.
Owner:SHENZHEN SHENTOU ENVIRONMENT TECH CO LTD
Recycling method of electroplating wastewater containing copper nitrate
ActiveCN103408164BShort processWide concentration rangeWater contaminantsWaste water treatment from metallurgical processCalcium hydroxideCopper nitrate
Owner:SHENZHEN SHENTOU ENVIRONMENT TECH CO LTD
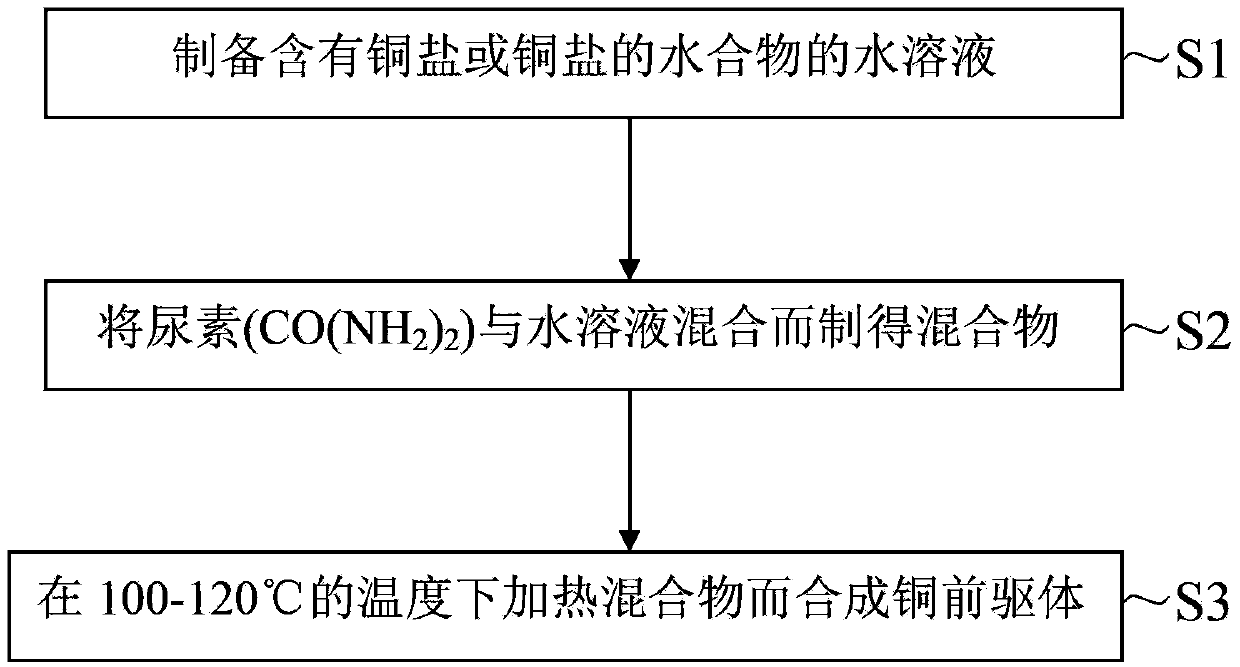
Method of producing a copper precursor and a copper precursor produced by using the same
InactiveCN103373740ATransportation and packagingMetal-working apparatusAqueous solutionMaterials science
There is provided a method of producing a copper precursor and a copper precursor produced by using the same. The method of producing a copper precursor includes: preparing an aqueous solution including a copper salt or a hydrate thereof; preparing a mixture by mixing urea (CO(NH2)2) with the aqueous solution; and synthesizing the copper precursor by heating the mixture at a temperature of 100 to 120° C.
Owner:SAMSUNG ELECTRO MECHANICS CO LTD
Method for producing basic metal nitrate
ActiveUS7462342B2Improve efficiencyHigh yieldNitrogen compoundsAlkali metal nitratesMetal nitrateNitrate
A process in which a basic metal nitrate is obtained in a high yield is provided.A process for producing a basic metal nitrate, which comprises adding an aqueous solution of a metal nitrate or an aqueous solution of a mixture of a metal nitrate and a water-soluble additive and an alkali solution to a reaction vessel in which a reaction solvent whose pH at 20° C. is adjusted to 6 or less is present, and conducting the reaction with stirring.
Owner:DAICEL CHEM IND LTD
Comprehensive utilization method of basic copper carbonate production waste liquid
ActiveCN103449501BAchieve reuseRealize recycling of resourcesCopper chloridesCopper nitratesSodium bicarbonateSulfate radicals
The invention discloses a comprehensive utilization method of waste liquor in the production of basic cupric carbonate and relates to the field of treatment methods of industrial wastewater. The invention aims at providing a comprehensive utilization method of waste liquor in the production of basic cupric carbonate, and in particular relates to a method for reusing sodium bicarbonate in the waste liquor. The waste liquor in the production of the basic cupric carbonate is the waste liquor generated after the basic cupric carbonate is produced by a reaction between an acidic copper chloride solution or an acidic copper sulfate solution and a sodium carbonate solution. The comprehensive utilization method of the waste liquor in the production of the basic cupric carbonate mainly comprises the following steps: adding a little distilled water into a reaction kettle as a base solution; when the reaction temperature rises to 35-90 DEG C, starting a stirring device, and adding the waste liquor containing sulfate radicals or chlorine and the acidic copper solution into the reaction kettle for reaction, wherein the pH value during the addition of the solutions is controlled to be 3-6; and filtering, washing, drying and sieving reaction products to obtain the basic copper salt. The method disclosed by the invention is simple to operate, easy to control reaction conditions, and widely applicable to the recycling and reusing of the waste liquor in the production of basic cupric carbonate.
Owner:SHENZHEN SHENTOU ENVIRONMENT TECH CO LTD
Method for producing nano basic copper nitrate
ActiveCN103193260BLarge Appearance ParticlesEasy to settleMaterial nanotechnologyCopper nitratesCombustionUltrafiltration
The invention discloses a method for producing nano basic copper nitrate. The method comprises the following steps of: (1) preparing the following reaction liquids: copper nitrate aqueous solution, caustic soda solution and ammonium nitrate solution, and performing ultrafiltration treatment on the copper nitrate aqueous solution and the caustic soda solution; (2) putting the ammonium nitrate solution in a reactor with an ultrasonic stirring device, and feeding the copper nitrate aqueous solution and the caustic soda solution which are subjected to ultrafiltration previously into the reactor for reaction via a mixed liquid high-pressure spraying device; (3) when the pH value in the reactor reaches 6.2-6.3, adding nitric acid and stopping adding until the pH value is in the range of 4.2-4.3; (4) further stirring for 40-60 minutes until the pH value is in the range of 4.3-4.5 and terminating the reaction to obtain a synthetic liquid; (5) standing the synthetic liquid, removing the clear liquid at the upper part in the tank and washing the precipitate using water; and (6) separating the obtained precipitate and drying, thereby obtaining the nano basic copper nitrate. The method is characterized in that the posttreatment process is simplified, the combustion and catalytic performance of the product are good and the application and popularization defects of the common copper nitrate are eliminated.
Owner:SANMING COFFER FINE CHEM IND
A kind of purification method of copper nitrate
ActiveCN104925848BAvoid it happening againWide variety of sourcesCopper nitratesPurification methodsCopper nitrate
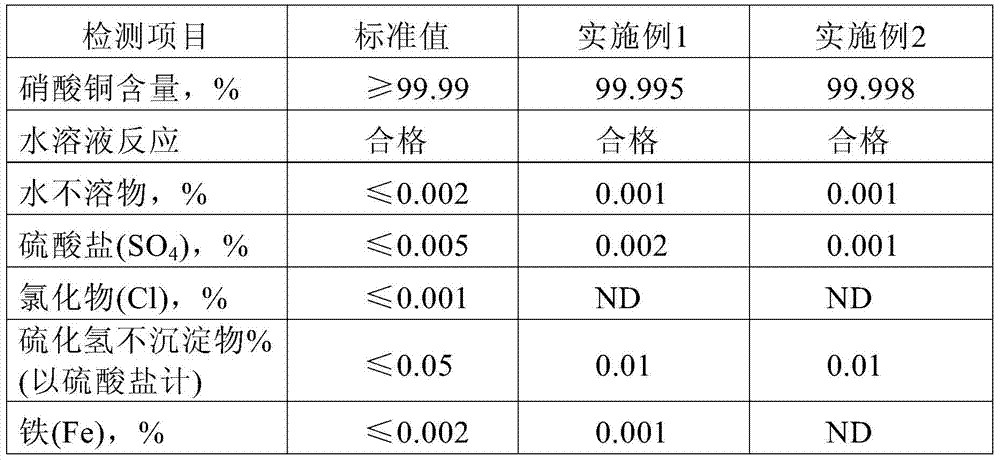
The invention discloses a copper nitrate purification method, comprising: taking industrial copper nitrate as a raw material; dissolving the industrial copper nitrate in high purity water with the specific impedance being greater than 17 Mohm.cm; adjusting the pH value by using high purity diluted nitric acid; adding in solid sodium sulfide; stirring the material at room temperature; keeping the material standing; performing filtering; making the filtrate run through a glass column loaded with weak base-strong acid ion exchange resin; performing distilling at the atmospheric pressure to remove water; performing cooling; and filtering and vacuum drying precipitated crystals to obtain purified copper nitrate. The measured value of the copper nitrate obtained from the purification method provided by the invention reaches 99.99%, with each indicator in line with copper nitrate spectrum pure 4N standard in the electronics industry. The invention has a good effect of impurity removal, has high efficiency, is safe and convenient in operation, has stable product quality and is suitable for industrial production.
Owner:SINOPHARM CHEM REAGENT
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com