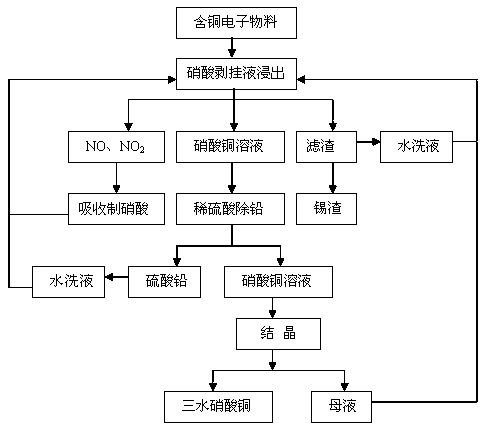
Method for preparing copper nitrate from nitric acid stripping liquid
A technology of stripping liquid and copper nitrate, which is applied in the direction of copper nitrate, lead sulfate, process efficiency improvement, etc., can solve the problems of high nitrate content, environmental pollution, large consumption, etc., and achieve low price, simple process and easy operation Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0045] Table 1 is the chemical composition of the waste electronic circuit board polymetallic powder used in Example 1:
[0046] Table 1 Chemical compositions of polymetallic powders from waste electronic circuit boards (%)
[0047] element
Cu
Fe
sn
Pb
Au
Ag
Al
Zn
W / %
73.22
1.12
10.1
8.71
0.001
0.03
6.25
0.57
[0048] (1) Leaching
[0049] Using nitric acid stripping liquid as raw material, add 200 kg of waste electronic circuit board polymetallic powder with the composition shown in Table 1 according to the liquid-solid ratio of 8:1, after reacting for 3 hours, filter and separate to obtain copper nitrate solution and filter residue, wash and dry SnO in the filter residue 2 The content is 65.28% (calculated as tin).
[0050] The composition of the copper nitrate solution is: Cu 2+ =82.56 / L,Pb 2+ =26.36g / L, Fe 3+ =3.5mg / L.
[0051] (2) Lead removal
[0052] Add the copper nitrate solution that s...
Embodiment 2
[0057] Table 2 is the copper rice chemical composition used in the embodiment two:
[0058] Table 2 Chemical composition of copper rice
[0059] element
Cu
Ni
sn
Pb
Al
Zn
W / %
88.26
1.12
6.1
2.32
1.25
0.95
[0060] (1) Leaching
[0061] Using nitric acid stripping liquid as raw material, add 300 kg of copper rice with the composition shown in Table 2 according to the liquid-solid ratio of 9:1. After reacting for 4 hours, filter and separate to obtain copper nitrate solution and filter residue. SnO in the filter residue after washing and drying with water 2 The content is 68.65% (calculated as tin).
[0062] The composition of the copper nitrate solution is: Cu 2+ =78.26 / L, Pb 2+ =21.65g / L.
[0063] (2) Lead removal
[0064] Add the copper nitrate solution that step 1 obtains to 1.5 times of theoretical amount of sulfuric acid, filter and wash after reacting for 3h, and the copper nitrate solution composition tha...
Embodiment 3
[0069] Table 3 is the chemical composition of the circuit board electronic pin waste used in Example 3:
[0070] Table 3 Chemical composition of circuit board electronic pin waste
[0071] element
Cu
Fe
sn
Pb
Al
Zn
W / %
59.6
13.12
16.3
9.25
0.78
0.95
[0072] (1) Leaching
[0073] Using nitric acid stripping liquid as raw material, add 500 kg of circuit board electronic pin waste with the composition shown in Table 3 according to the liquid-solid ratio of 10:1. After reacting for 5 hours, filter and separate to obtain copper nitrate solution and filter residue, wash and dry the filter residue SnO 2 The content is 63.26% (calculated as tin).
[0074] The composition of the copper nitrate solution is: Cu 2+ =76.25 / L, Pb 2+ =21.25g / L.
[0075] (2) Lead removal
[0076] Add the copper nitrate solution that step 1 obtains to 1.3 times of theoretical amount of sulfuric acid, filter and wash after reacting for 3h, and...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com