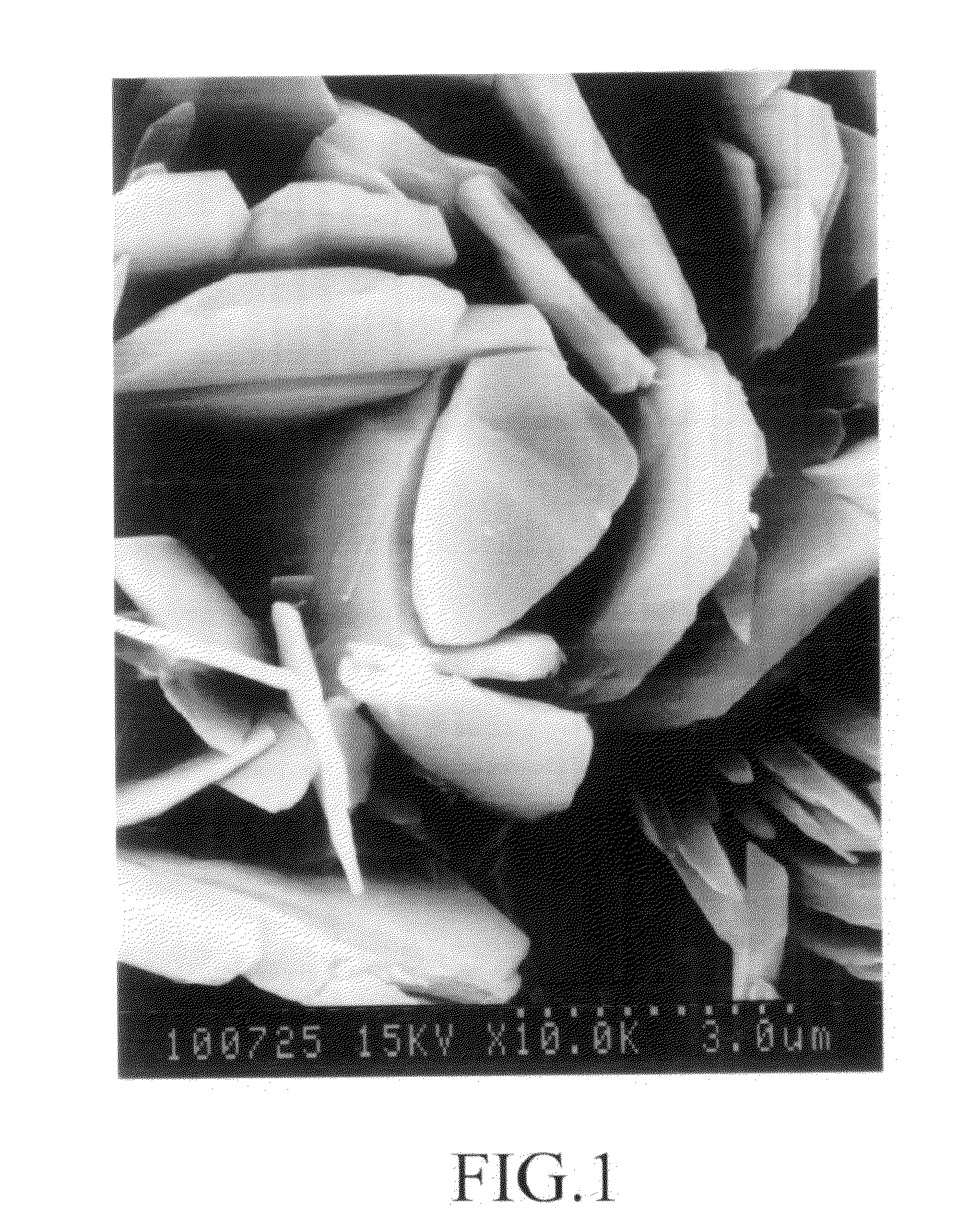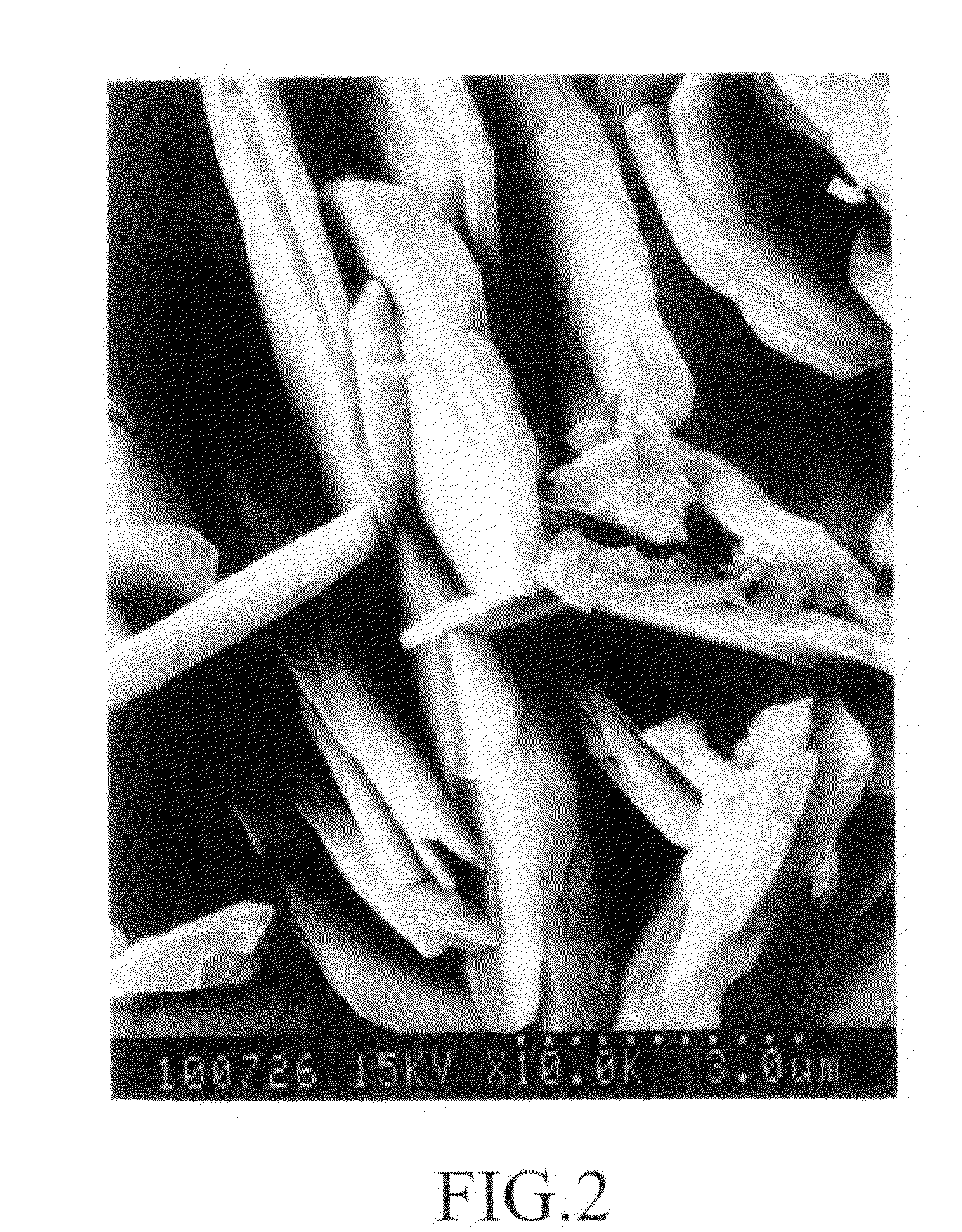Basic metal nitrate, process for producing the same and gas generating agent composition
a technology of metal nitrate and gas generating agent, which is applied in the direction of weapons, chemistry apparatus and processes, explosives, etc., can solve the problems of toxicity to human bodies or hazards in the handling of sodium azide, and achieve the effect of high process for producing the sam
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
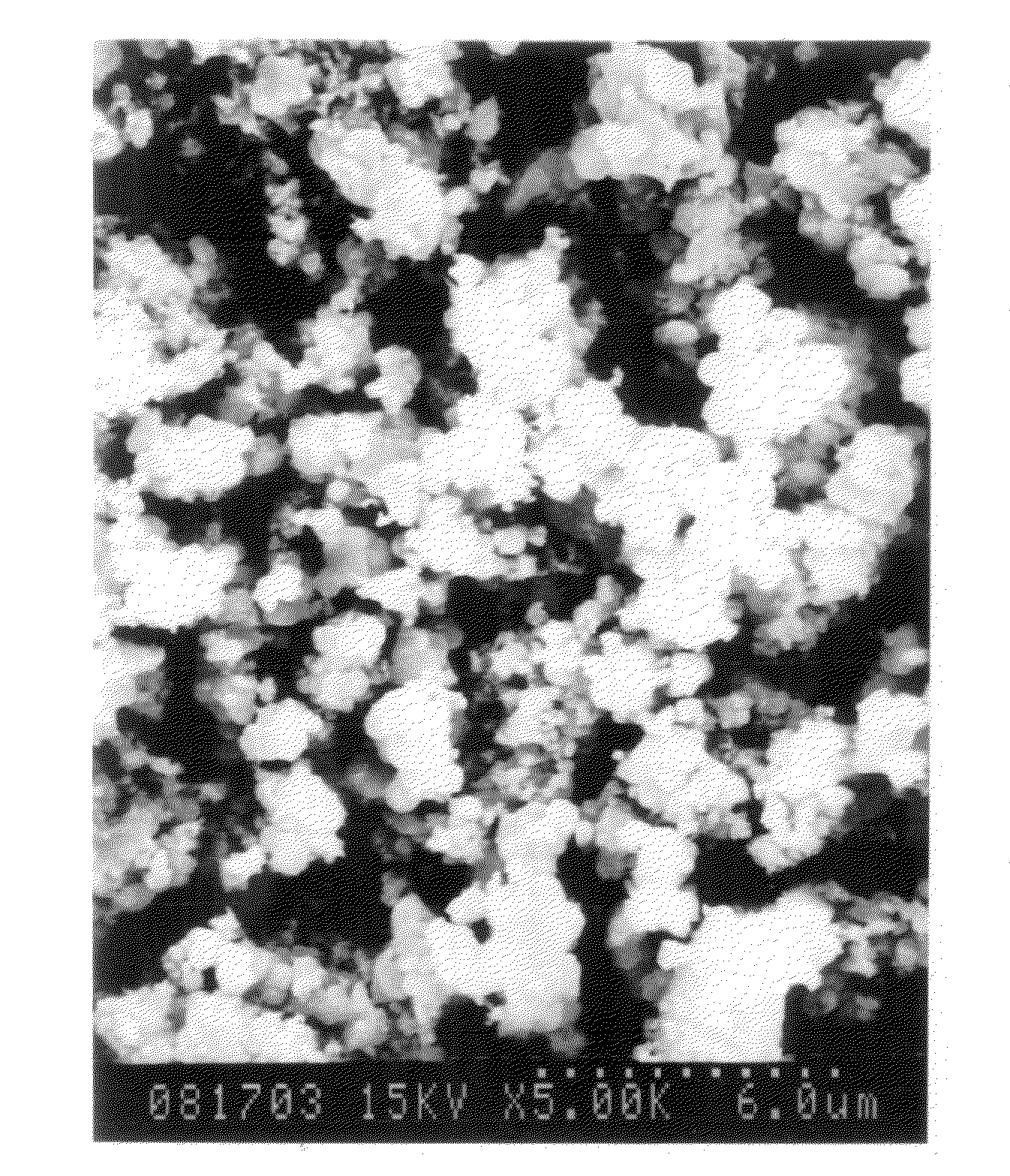
[0204]36.3 g of copper nitrate 3-hydrate was charged in a beaker fitted with a stirrer, and then dissolved in 100 ml of distilled water which was poured therein, while being stirred. The resulting solution was heated at 60° C. A solution of 18.9 g of sodium bicarbonate in 240 ml of water was added over 1 hour. After the addition was completed, the mixture was aged while being stirred at 60° C. for 60 minutes. The resulting gel-like precipitate was filtered at room temperature, and washed with distilled water. A pale blue solid matter having quite a good filterability was obtained. When a part of the washed product was dried in air at 110° C., the product maintained the pale blue color, and the thermal stability was quite good. The remaining washed product was dried at 110° C. under reduced pressure of 1,333.22 Pa to obtain a basic copper nitrate in an amount of 17.4 g (yield 96.5%). The results of measurements are shown in Table 1.
example 2
[0205]36.3 g of copper nitrate 3-hydrate was charged in a beaker fitted with a stirrer, and then dissolved in 100 ml of distilled water which was poured therein, while being stirred. The resulting solution was heated at 80° C. A solution of 18.9 g of sodium bicarbonate in 240 ml of water was added over 1 hour. Immediately after the addition was completed, a precipitate was filtered, and washed with distilled water to obtain a pale blue solid matter having quite a good filterability. When a part of the washed product was dried in air at 110° C., the product maintained the pale blue color, and the thermal stability was good. The remaining washed product was dried at 110° C. under reduced pressure of 1,333.22 Pa to obtain a basic copper nitrate. The results of measurements are shown in Table 1.
example 3
[0206]214.6 g (1.00 mol) of copper nitrate 3-hydrate was charged in a beaker fitted with a stirrer, and then dissolved in 500 ml of distilled water which was poured therein, while being stirred. The resulting solution was heated at 40° C. A solution of 126 g (1.50 mols) of sodium bicarbonate in 1,000 ml of distilled water was added over 1 hour. After the addition of sodium bicarbonate was completed, the mixture was heated to 8° C., and aged for 30 minutes while being stirred. A precipitate was filtered, washed, and dried to obtain a pale blue basic copper carbonate. The results of measurements are shown in Table 1.
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| internal pressure | aaaaa | aaaaa |
| TG- | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com