A kind of purification method of copper nitrate
A purification method, copper nitrate technology, applied in the direction of copper nitrate, etc., can solve the problems of high raw material requirements, complicated production process, etc., and achieve the effects of wide sources, stable product quality, safe and easy operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] Dissolve 100g of industrial copper nitrate in 500ml of high-purity water with a specific impedance greater than 17MΩ·cm, adjust to pH 2 with 5wt% high-purity dilute nitric acid, add 2g of solid sodium sulfide, stir for 1 hour at room temperature, and filter after standing. The filtrate is passed through a glass column loaded with weak base-strong acid ion exchange resin D511 (the weak base ion exchange group is primary amine, and the strong acid ion exchange group is sulfonic acid group.), and the water is distilled off under normal pressure, cooled At 10°C, the precipitated crystals were filtered and vacuum-dried at a temperature of 40°C for 12 hours to obtain 91 g of purified copper nitrate.
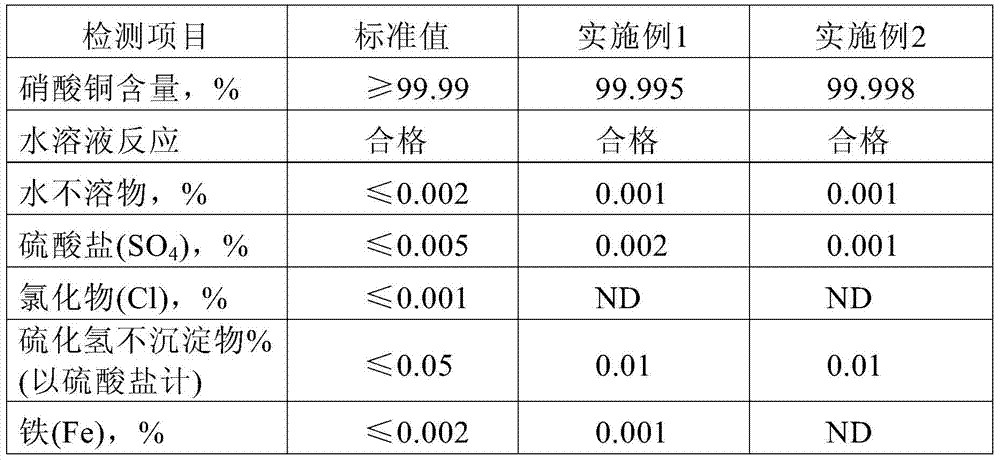
[0024] The test results show that all the indicators of the product meet the standard spectroscopically pure 4N standard (copper nitrate used in the electronics industry), and the specific test data are shown in Table 1.
Embodiment 2
[0026] Dissolve 100g of industrial copper nitrate in 1000ml of high-purity water with a specific impedance greater than 17MΩ·cm, adjust to pH 3 with 10wt% high-purity dilute nitric acid, add 5g of solid sodium sulfide, stir for 2 hours at room temperature, and filter after standing. Pass the filtrate through a glass column loaded with weak base-strong acid ion exchange resin D512 (the weakly basic ion exchange group is a tertiary amine, and the strongly acidic ion exchange group is a sulfonic acid group.), and distill off moisture under normal pressure , cooled to 5° C., filtered the precipitated crystals, and dried in vacuum at a temperature of 60° C. for 8 hours to obtain 88 g of purified copper nitrate.
[0027] The test results show that all the indicators of the product meet the standard spectroscopically pure 4N standard (copper nitrate used in the electronics industry), and the specific test data are shown in Table 1.
[0028] Table 1
[0029]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com