Method for preparing benzene end-capping polyaryletherketone polyme
A polyaryletherketone and polymer technology, which is applied in the synthesis field of polyaryletherketone, can solve the problems such as inability to carry out continuous production, inaccurate final capping dosage, and unsuitability for industrial production.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
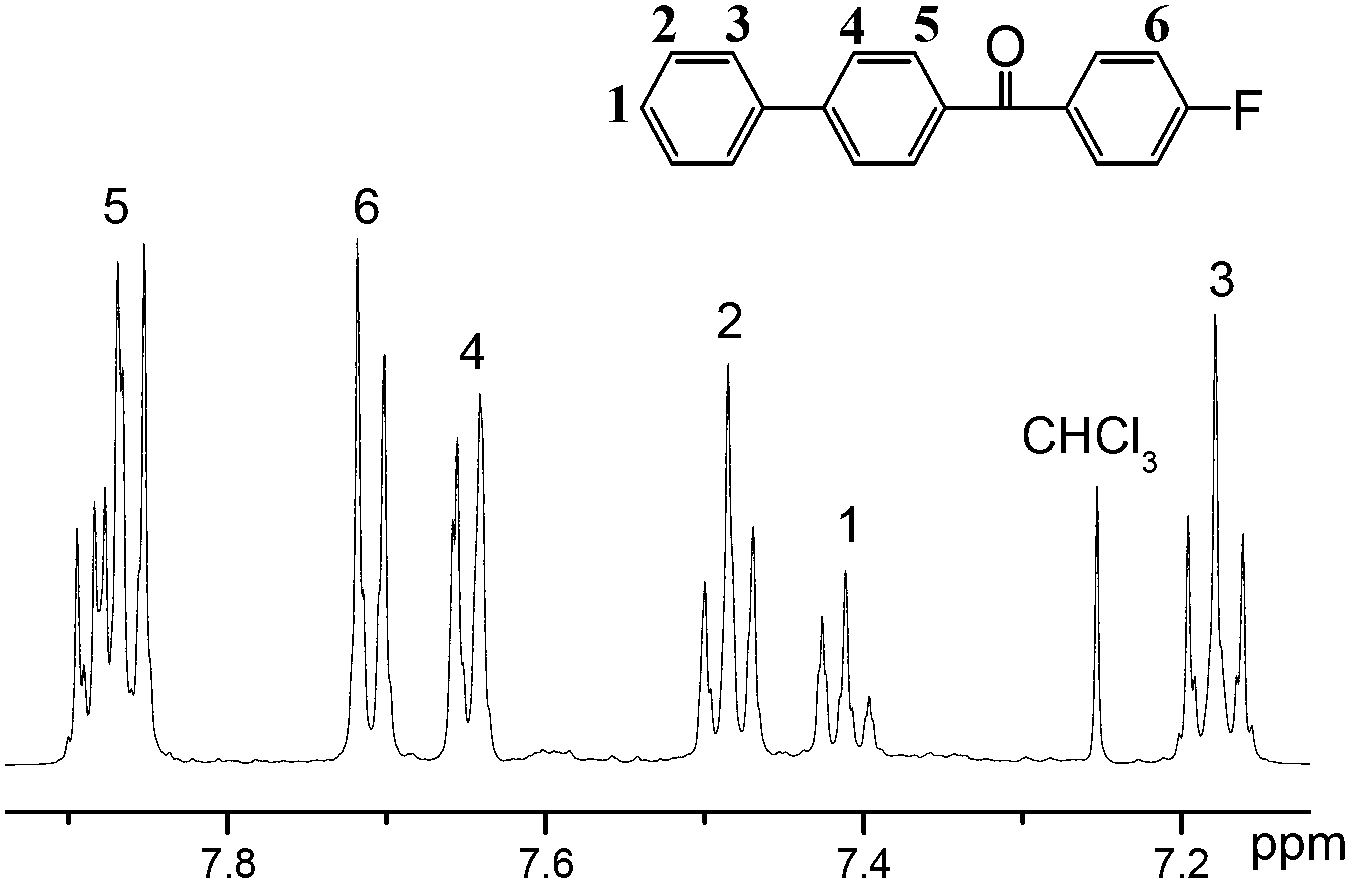
[0033] Add 154.21g of biphenyl and 450ml of dichloromethane into the three-necked flask, stir until the former is completely dissolved in the latter, then cool the system down to about 0°C, slowly add 133.50g of aluminum trichloride, and stir until no bubbles come out. After fully mixing 158.56g of p-fluorobenzoyl chloride and 50ml of dichloromethane, slowly add it dropwise to the above reaction system, stir slowly for 0.5 hours, then raise the temperature to about 20°C and stir for about 5 hours, then pour the reactant into ice hydrochloric acid aqueous solution , fully shaken, poured out the water, washed 4 times with hydrochloric acid aqueous solution, evaporated dichloromethane to dryness to obtain a solid, and recrystallized from a mixed solution of dichloromethane and ethanol (volume ratio 1:1) to obtain 4- Fluorobenzoyl diphenyl ether 192.04g. Its NMR spectrum is as figure 1 shown.
Embodiment 2
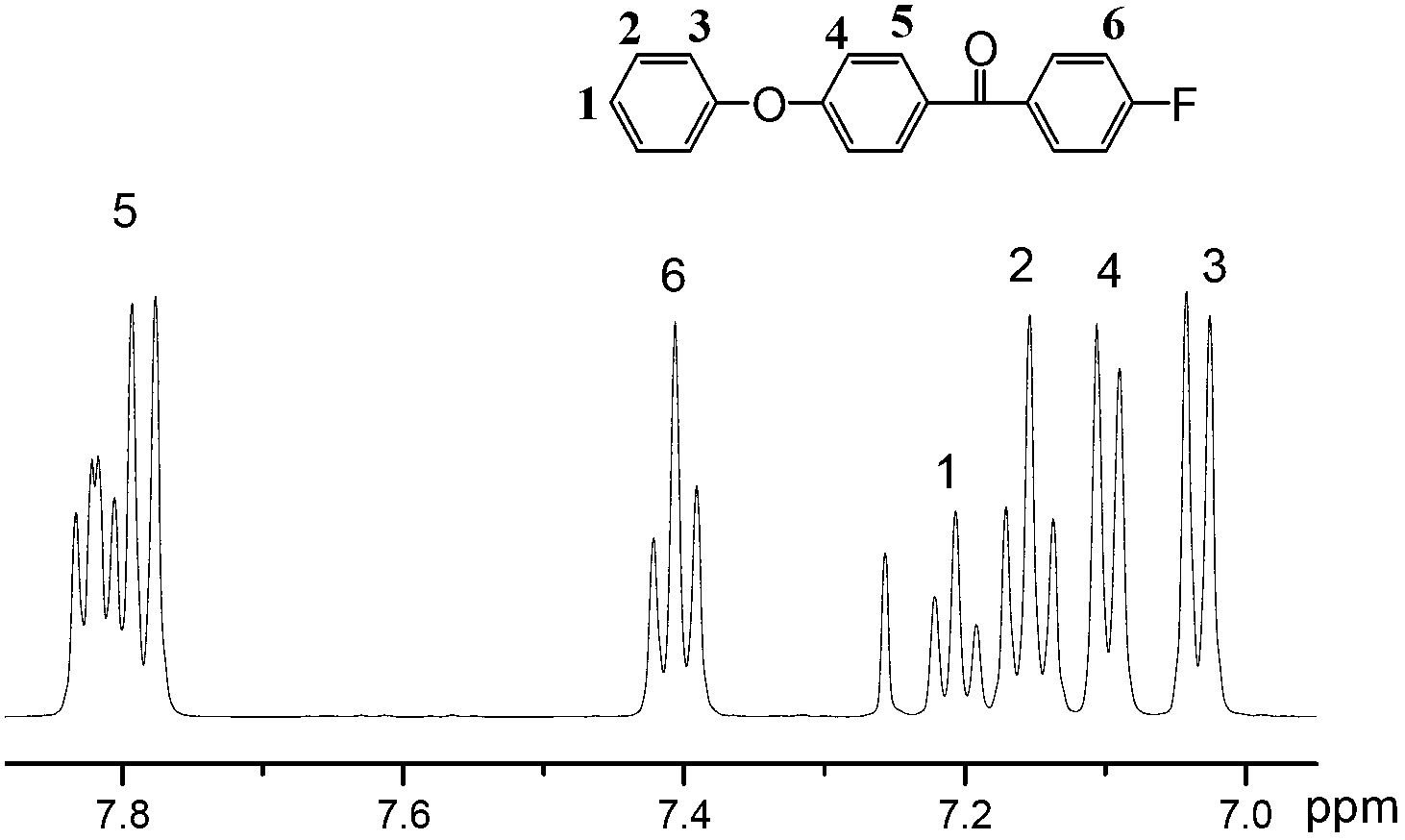
[0035]Add 170.21g of diphenyl ether and 450ml of dichloromethane into the three-necked flask, stir until the former is completely dissolved in the latter, then cool the system down to about 0°C, slowly add 133.50g of aluminum trichloride, and stir until no bubbles come out. After fully mixing 158.56g of p-fluorobenzoyl chloride and 50ml of dichloromethane, slowly add it dropwise to the above reaction system, stir slowly for 0.5 hours, then raise the temperature to about 20°C and stir for about 5 hours, then pour the reactant into ice hydrochloric acid aqueous solution , fully shaken, poured out the water, washed 4 times with aqueous hydrochloric acid, evaporated dichloromethane to dryness to obtain a solid, and recrystallized from ethanol to obtain 263 g of 4-fluorobenzoyl diphenyl ether as a white solid. Its NMR spectrum is as figure 2 shown.
Embodiment 3
[0037] Add 40.4g of 4,4'-difluorodiphenyl ketone and 146g of diphenyl sulfone (30% solid content) into a three-neck flask equipped with mechanical stirring and a thermometer, under nitrogen protection, heat to 160°C, and then add anhydrous Potassium carbonate 1.41g and anhydrous sodium carbonate 21.17g, then heated to 165°C, add hydroquinone 30.43g, continue to heat to 200°C and control the temperature for 1 hour, gradually raise the temperature to 250°C for 15 minutes, and then control the temperature at 280°C 1 hour, finally heated to 320°C for 3 hours, added 1.42 g of 4-(p-fluorobenzoyl)biphenyl and reacted for 1 hour, poured the product into water, crushed with a pulverizer, filtered, and directly boiled the solid with ethanol or acetone Boil and filter by the same method, repeat 5-6 times, then boil with distilled water, filter, repeat 5-6 times, dry in an oven to obtain refined polymer. The yield is 97%, the glass transition temperature is 147°C, and the melting point is...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Glass transition temperature | aaaaa | aaaaa |
| Melting point | aaaaa | aaaaa |
| Melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com