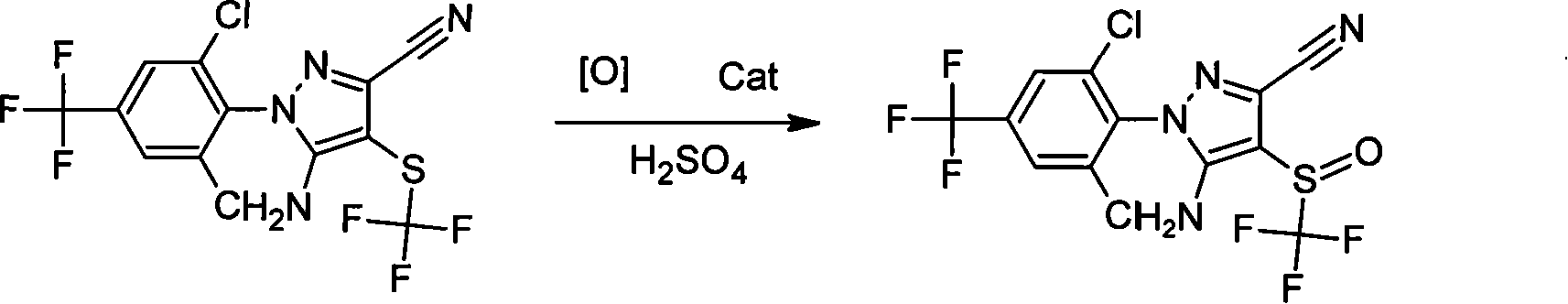
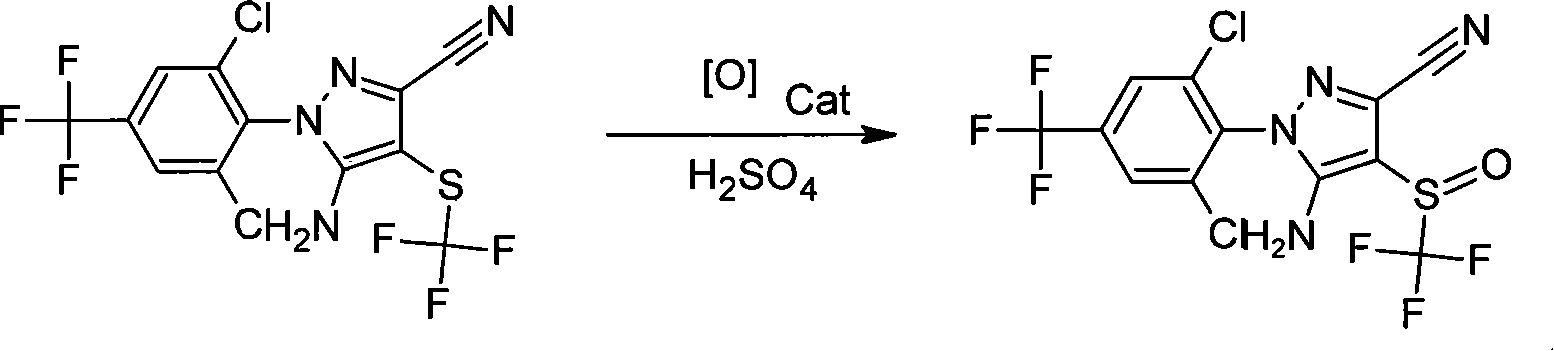
Preparation method of fipronil
A technology of fipronil and trifluoromethyl phenyl is applied in the field of oxidizing 5-amino-3-cyano-1--4-trifluoromethylthiopyrazole to generate fipronil, which can solve the problem of expensive trifluorocarbon Acetic acid and trifluoroacetic acid cannot be directly applied, etc., to achieve the effects of simple process, good industrial application prospects and environmental friendliness
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0015] Under cooling, add 40 grams of 5-amino-3-cyano-1-(2,6-dichloro-4-trifluoromethylphenyl)-4-trifluoromethylthiopyrazole, 200ml of three-necked flask Methyl chloride, stir for 20 minutes, add 40 grams of 118% sulfuric acid, 1 gram of tetrabutylammonium chloride, slowly add 16 grams of 30% hydrogen peroxide, control the temperature -10~-5℃, and react at this temperature 5 After ~6 hours, sample and monitor. After the reaction is completed, 120ml of water is added dropwise at 0~4℃, and the layers are separated. The organic layer was washed with saturated sodium bicarbonate solution until it was neutral, separated into layers, desolvated, and dried to obtain 39.2 g of fipronil technical.
Embodiment 2
[0017] Under cooling, 24 grams of sodium perborate and 0.4 grams of tetrabutylammonium bromide were slowly added to 80 grams of 96% sulfuric acid, stirred for 20 minutes, and 40 grams of 5 dissolved in 200 ml of dichloromethane were added dropwise at 0-4°C. -Amino-3-cyano-1-(2,6-dichloro-4-trifluoromethylphenyl)-4-trifluoromethylthiopyrazole, and react at this temperature for 2 to 3 hours, and sample After monitoring, after the reaction is completed, 80 ml of water is added dropwise at 0-4°C, and the layers are separated. The organic layer was washed with saturated sodium bicarbonate solution to neutrality, separated into layers, desolvated, and dried to obtain 39.3 g of fipronil technical.
Embodiment 3
[0019] Under cooling, 20 grams of sodium perborate and 1 gram of 4-N,N-lutidine were slowly added to 400 grams of 60% sulfuric acid, stirred for 20 minutes, and then added dropwise to 50 ml of carbon tetrachloride at 10°C. 40 grams of 5-amino-3-cyano-1-(2,6-dichloro-4-trifluoromethylphenyl)-4-trifluoromethylthiopyrazole, and react at this temperature for 6-8 After the reaction is completed, 60ml of water is added dropwise at 10°C, and the layers are separated. The organic layer was washed with saturated sodium bicarbonate solution to neutrality, separated into layers, desolvated, and dried to obtain 39.4 g of fipronil technical.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com