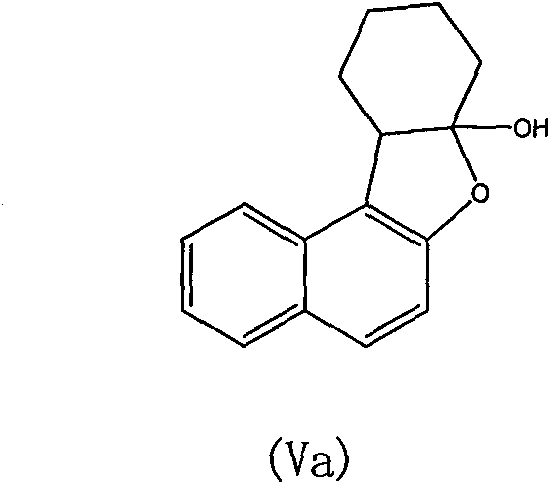
Preparation method of naphthonone and analogues thereof
A technology of analogue and naptolone, which is applied in the field of compound preparation, can solve the problems of complex reaction conditions, troublesome post-processing, high production cost, etc., and achieve the effects of simple post-processing, less reaction by-products and low production cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
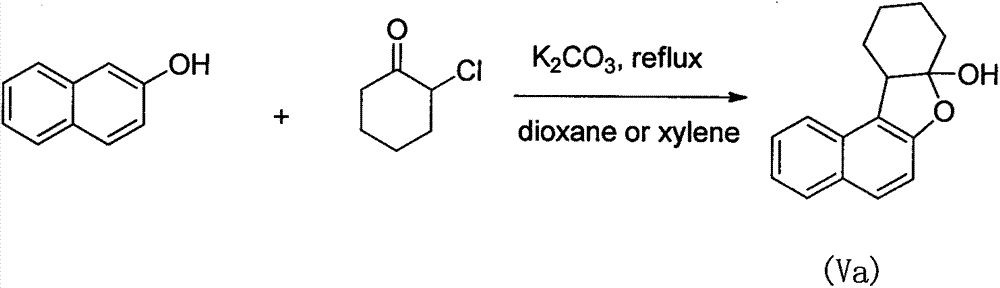
[0031] Embodiment 1: 2-naphthol reacts with 2-chlorocyclohexanone to generate naptolone
[0032]
[0033] Sodium carbonate (0.15mol), 2-naphthol (0.1mol) and 150mL trifluoroethanol solvent were added respectively in a 500mL round bottom flask, then 2-chlorocyclohexanone (0.11mol) was dissolved in 50mL trifluoroethanol solvent, Slowly drop into a round bottom flask through a constant pressure dropping funnel. The reaction mixture was stirred at room temperature for 12 h. After the TCL monitors the reaction, the inorganic salts are removed by filtration. The filtrate was distilled under reduced pressure to recover the trifluoroethanol solvent to obtain the crude product as off-white solid. Further purification and recrystallization with ethanol gave colorless crystalline compound (Va), namely naptolone (0.091 mol), with a yield of 91%. Naptolone: Mp: 136-137°C; 1 H NMR (400MHz, CDCl 3 )δ7.81(d, J=8.3Hz, 1H), 7.69(t, J=9.2Hz, 2H), 7.45(ddd, J=8.2, 6.9, 1.2Hz, 1H), 7.31(...
Embodiment 2
[0034] Embodiment 2: 2-naphthol reacts with 2-bromocyclohexanone to generate naptolone
[0035]
[0036]In a 500mL round bottom flask, add triethylamine (0.2mol), 2-naphthol (0.1mol) and 150mL hexafluoroisopropanol solvent, then dissolve 2-bromocyclohexanone (0.15mol) in 50mL hexafluoro The isopropanol solvent was slowly dropped into the round bottom flask through the constant pressure dropping funnel. The reaction mixture was stirred at room temperature for 8 h. After the reaction was monitored by TCL, the solid mixture was obtained by distillation under reduced pressure, then dissolved in water, and extracted with dichloromethane. The extract was distilled under reduced pressure to obtain the primary product, which was further purified and recrystallized from ethanol to obtain colorless crystalline compound (Va), namely naptolone (0.090 mol), with a yield of 90%.
Embodiment 3
[0037] Embodiment 3: 2-naphthol reacts with 2-chloro-6-methylcyclohexanone to generate compound (Vb)
[0038]
[0039] Potassium carbonate (0.15mol), 2-naphthol (0.1mol) and 150mL of hexafluoroisopropanol were added to a 500mL round bottom flask, and then 2-chloro-6-methylcyclohexanone (0.15mol) was dissolved Slowly drop 50 mL of hexafluoroisopropanol into a round bottom flask through a constant pressure dropping funnel. The reaction mixture was stirred at room temperature for 8 h. After the TCL monitors the reaction, the inorganic salts are removed by filtration. The filtrate was distilled under reduced pressure to recover the hexafluoroisopropanol solvent to obtain a brown solid crude product, which was then purified by silica gel column chromatography using a mixed solvent of petroleum ether and ethyl acetate (1:1 by volume) as the eluent to obtain the compound (Vb) (0.089 mol), 73% yield. 1 H NMR (400MHz, CDCl 3 )δ7.80(d, J=8.2Hz, 1H), 7.69(d, J=8.8Hz, 2H), 7.48-7.4...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com