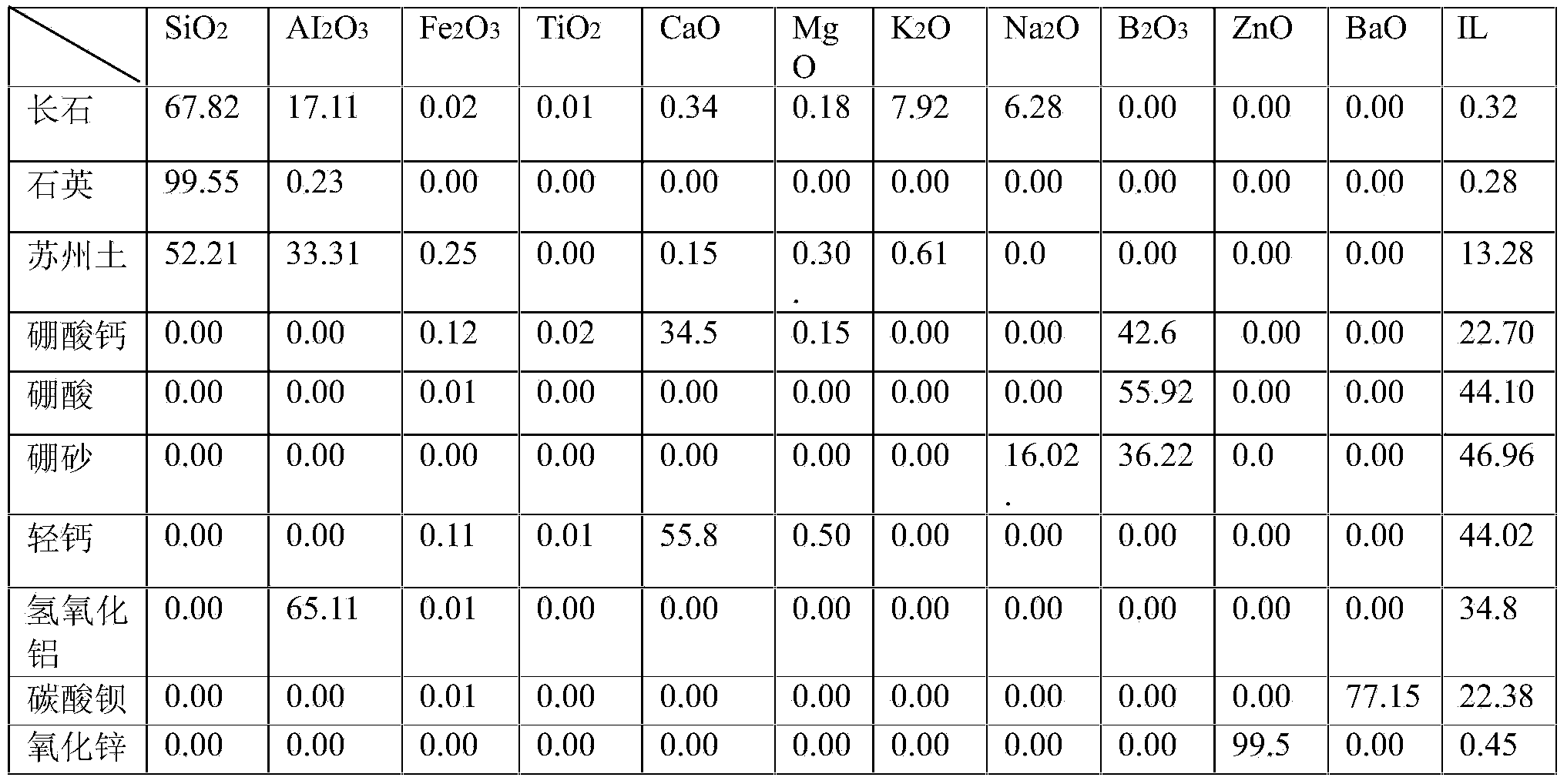Patents
Literature
373 results about "Calcium borate" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Calcium borate (Ca₃(BO₃)₂), also called Gerstley borate, is a bluish white crystal with a very defined structure. It can be prepared by reacting calcium metal with boric acid. The resulting precipitate is calcium borate. A hydrated form occurs naturally as the minerals colemanite, nobleite and priceite.
Mixed solubility borate preservative
InactiveUS7449130B2Reduce solubilityImprove solubilityBiocideOther chemical processesCelluloseSolubility
Preservative composition for lignocellulosic-based composites providing rapid and long-lasting protection against insect and fungal attack, in both low and high moisture environments, through the use of a combination of higher solubility and lower solubility borates. Pesticidal amounts of a lower solubility borate and a higher solubility borate are combined before or during their incorporation into a lignocellulosic-based composite. Useful low solubility borates includes copper borate, zinc borate and barium metaborate. Useful high solubility borates includes boric acid, boric oxide, ammonium borate and alkali metal borates such as sodium borate. Some alkaline earth metal borates, including calcium borates, are of intermediate solubility and may be used effectively as either a low soluble or high soluble borate depending on the combination used.
Owner:U S BORAX INC
Transparent lead-free fritted glaze with low expansion coefficient and preparation method thereof
The invention relates to a transparent lead-free fritted glaze with low expansion coefficient and a preparation method thereof. The fritted glaze comprises the following chemical ingredients of: 59 to 66 percent of SiO2, 10 to 15 percent of Al2O3, 5 to 10 percent of B2O3, 3 to 6 percent of CaO, 4 to 8 percent of MgO, 0 to 3 percent of K2O, 0 to 2 percent of Na2O, 0 to 1 percent of Li2O, 1 to 5 percent of ZnO and 0 to 2 percent of SrO; and the fritted glaze comprises the raw materials of: 10 to 25 percent of kaolin, 15 to 30 percent of quartz, 10 to 25 percent of potassium feldspar, 0 to 8 percent of dolomite, 2 to 6 percent of grammite, 10 to 20 percent of roasted talc, 1 to 6 percent of zinc oxide, 2 to 8 percent of boric acid, 5 to 15 percent of calcium borate, 1 to 5 percent of alumina, 0 to 2 percent of lithium carbonate and 0 to 3 percent of strontium carbonate. The expansion coefficient of the fritted glaze is 3.7-4.5*10-6 / DEG C (RT to 500 DEG C), the melted temperature is 1400 DEG C to 1500 DEG C, the glaze firing temperature is 1130 DEG C to 1230 DEG C; and the transparent lead-free fritted glaze has fine and bright glaze surface, high transparency, low expansion coefficient as well as no lead precipitation and is particularly suitable for double-fired hard porcelain body with lower expansion coefficient.
Owner:JINGDEZHEN CERAMIC INSTITUTE
High and low-temperature resistant porcelain glaze for enamel
InactiveCN103274764AImprove smoothnessGood high and low temperature resistanceTemperature resistanceLead sulfate
The invention relates to porcelain glaze and in particular relates to high and low-temperature resistant porcelain glaze for enamel. According to the technical scheme, the highnd low-temperature resistant porcelain glaze for enamel is prepared from the following raw materials in parts by weight: 22-38 parts of sodium silicate with the modulus of 2-3.8, 11-19 parts of aluminum oxide, 10-20 parts of manganese dioxide, 6-9 parts of palladium oxide, 6-9 parts of high-boron calcium borate, 23-29 parts of lead sulfate, 2-4 parts of talc, 7-13 parts of calcium carbonate, 8-12 parts of barium sulfate, 12-19 parts of zinc sulfate and 2-9 parts of titanium oxide. The high and low-temperature resistant porcelain glaze has excellent ultralow-temperature resistance and high-temperature resistance.
Owner:XIANGFENG ZHEJIANG METAL PROD
Compound grease of calcium sulfonic acid, and preparation method
This invention relates to composite lubricating grease, which comprises: calcium sulfonate with a base number of 250-320 mgKOH / g 30-60 wt. %, calcium borate 0.8-5.1 wt. %, C12-C24 fatty acid calcium soap 1.0-6.8 wt. %, and lubricating base oil. The composite lubricating grease has such advantages as low cost, good synthetical properties, high dropping point, high colloid stability and good water proof property.
Owner:CHINA PETROLEUM & CHEM CORP +1
Lignocellulosic composites
InactiveUS7163974B2Reduce environmental problemsEasy to handleBiocideAnimal repellantsCelluloseCalcium borate
Lignocellulosic-based woodfiber-plastic composite products containing a pesticidal amount of calcium borate is resistant to attack by wood destroying fungi and insects. The preferred calcium borates are the calcium polytriborates having a CaO:B2O3 molar ratio of about 2:3 and calcium hexaborates, having a CaO:B2O3 ratio of 1:3. Composites can be produced by combining the calcium borate with particles of the lignocellulosic material and the thermoplastic resin binder, and heating and extruding the resultant mixture through a die to form the composite product.
Owner:U S BORAX INC
Method for comprehensively modifying saline and alkaline land
ActiveCN102172116AHighlight substantiveHighlight substantive featuresSoil lifting machinesOrganic fertilisersAlkali soilMicrobial agent
The invention relates to a method for comprehensively modifying saline and alkaline land. The method comprises the following steps: preparing a saline and alkaline land modifier from 50-80kg of 95wt% (percent by weight) potassium sulfate, 30kg of 98wt% zinc sulfate, 30kg of 95wt% aluminum sulfate, 20kg of 95wt% manganese sulfate, 20kg of calcium chelating agent, 40kg of 95wt% calcium borate, 100kg of microbial agent, 2,000kg of crop straw with the length of 3-10cm and 300-500kg of 39wt% humic acid; leveling soil, restoring ridges and digging ditches when the temperature is constantly stable and is over 15 DEG C; uniformly scattering the saline and alkaline land modifier on the surface of the land; turning up the soil so that the saline and alkaline land modifier is uniformly distributed in the deep soil layer of 0-20cm below the surface of the land; irrigating the land with water depth of 5cm for about 10 consecutive days; transplanting saline and alkaline resisting rice seedlings to the modified saline and alkaline land; applying a small amount fertilizer for multiple times without changing the total amount of the fertilizer; and keeping the water in the land shallow.
Owner:吉林省汇泉农业科技有限公司
Brilliant iron rust glaze, brilliant iron rust glaze ceramic product prepared from same and preparation method
The invention relates to a brilliant iron rust glaze, a brilliant iron rust glaze ceramic product prepared from the same and a preparation method, and belongs to the technical field of ceramics. The brilliant iron rust glaze comprises components in parts by mass as follows: 40-50 parts of nepheline-syenite, 10-20 parts of quartz, 2-8 parts of calcium carbonate, 18-22 parts of low-temperature frits, 2-6 parts of zinc oxide. 8-13 parts of calcium borate, 18-23 parts of manganese oxide and 1-4 parts of copper oxide, wherein the low-temperature frits comprise components in parts by mass as follows: 44-47 parts of borax, 28-32 parts of quartz, 18-22 parts of potash feldspar and 2-7 parts of kaolin. The brilliant iron rust glaze can imitate the iron rust appearance, so that the ceramic product can show the iron rust appearance on the surface and has brilliant silver bright points with three-dimensional sense; besides, with the adoption of the method, control is facilitated in a sintering process, energy saving is facilitated, and the yield of sintered products is higher.
Owner:FUJIAN DEHUA HUAMAO CERAMICS CO LTD
Boric fertilizer water dispersing granule and preparing method thereof
InactiveCN101531550AFast disintegrationDissolve (disperse) completelyFertiliser formsFertilizer mixturesMagnesium phosphatePotassium
The invention belongs to a new boric fertilizer, referring to a boric fertilizer water dispersing granule and preparing method thereof. The invention comprises one or more than one boric fertilizers and at least one surfactant; the materials are processed as the regular or irregular granules water dispersing granule; the weight part of the boric fertilizer is 5-95 parts; the rest is the assistant. The preferable solution of the invention is following: the boric fertilizer comprises one of the boric acid, boron oxide, boric acid ammonium, sodium tetraborate, calcium borate side, partial sodium borate, sodium borate tetrahydrate, magnesium diboride, sodium perborate, zinc borate, boric acid manganese, high-sodium borate, partial acid potassium, boron and magnesium fertilizer, boron and magnesium phosphate and boron mud or the mixture of the two or more than two of the above. The preparing method of the invention is scaling the prescription, mixing, crushing, and granule and drying to obtain the product. The boric fertilizer water dispersing granule of the invention has a fast disintegration, dissolving (dispersing) completely, not plugging the nozzle and the dropping pipe lines, no heating while using, easy to absorb moisture, packaging, storage and transportation, measurement, the use of low cost, widely used in food crops, cash crops, horticulture, lawns and urban greening; the market prospect is very broad.
Owner:SHENZHEN LANGTAI BIOTECH
Coke passivating agent
InactiveCN101654634AImprove thermal performanceInhibition of lysis reactionSolid fuelsBlast furnace detailsSilicon dioxideCalcium EDTA
The invention discloses a coke passivating agent. Raw materials of the coke passivating agent comprises the following components in percentage by weight: 1-16 glucose or calcium chloride, 1-45 boron anhydride, 1-15 titanium pigment, 1-10 silicon dioxide, 1-45 anhydrous borax, 1-46 calcium borate and 1-30 barium metaborate. The coke passivating agent can favorably improve the thermal performance and the thermal intensity of coke, inhibit the solution loss reaction of the coke, lower the reactivity of the coke, optimize a form of carbon and reduce the breakage of the coke fed into a furnace. The coke using the coke passivating agent is used for melting iron in a blast furnace and can increase the yield by 1-4 percent and save coke by 8-20 kg / t.iron. The industrialization of the product is beneficial to propelling the adjustment of an energy source industrial structure of China and improving the quality of the coke, strengthens the market competitiveness of a steel enterprise, has realistic meanings on aspects of economy, resources and environments and conforms to a sustainable development strategy.
Owner:重庆汉砧科技有限公司
Fluoride-free mould flux for continuous casting of zirconium-containing medium-carbon steel
The invention discloses a fluoride-free mould flux for continuous casting of zirconium-containing medium-carbon steel. The mould flux is composed of, by mass, 35%-45% of CaO, 30%-35% of SiO2, 2%-5% of Al2O3, 1%-3% of MgO, 6%-10% of B2O3, 10%-15% of (Na2O+Li2O) and 1%-3% of ZrO2, wherein the ratio of (Na2O+Li2O+ZrO2) to B2O3 is greater than or equal to 1.3 and smaller than or equal to 2. The fluoride-free mould flux is excellent in crystallization property, the crystallized phase is calcium borate silicate, and meanwhile ZrO2 is added so as to facilitate precipitation of the calcium borate silicate, refine grains of the calcium borate silicate and enhance the stability of the calcium borate silicate. The mould flux contains no fluoride, solves the problem of fluorine pollution in the continuous casting process, and can greatly reduce treatment cost of continuous casting cooling water and the corrosion speed of a casting machine.
Owner:CENT SOUTH UNIV
Method for growing calcium borate oxysalt crystal with frequency multiplication effect
InactiveCN101942699AEasy to processSimplify the Orientation ProgramPolycrystalline material growthBy pulling from meltCalcium borateX-ray
The invention relates to a method for growing a calcium borate oxysalt crystal with a frequency multiplication effect. The method comprises the following steps of: weighing raw materials according to a stoichiometric ratio of a chemical equation of the calcium borate oxysalt and performing crystal growth by the traditional pulling method, wherein a seed crystal is formed by finding the direction of the maximum nonlinear coefficient of the calcium borate oxysalt crystal which grows in the traditional direction through X-ray diffraction and cutting the crystal along the optimum phase matching direction; conveying the cut seed crystal slowly, vertically and downwards into polycrystal material melt to make the top of the seed crystal contact with the polycrystal material melt; maintaining the seed crystal to grow in the vertical direction of the polycrystal material liquid level of the calcium borate oxysalt; and obtaining the calcium borate oxysalt crystal with the frequency multiplication effect through three technological processes of necking, shouldering and ending. The method avoids the defects of complicated process, low utilization ratio, low properties of devices and the like caused by that the traditional calcium borate oxysalt crystal grows along the b direction. The method has the advantages of high optical homogeneity in the light-transmitting direction and high practical value.
Owner:SHANDONG UNIV +1
Light-weight, heat-insulating and environment-friendly composite wall building material
InactiveCN105801063AImprove flexural strengthHigh compressive strengthCeramicwareLow-density polyethyleneCellulose
The invention discloses a light-weight, heat-insulating and environment-friendly composite wall building material.The building coating is prepared from cellulose, carbon fiber particles, rubber particles, plant particles, low-density polyethylene resin, organosilicon-modified epoxy resin, calcium carbonate, magnesium oxide, magnesium silicate, magnesium calcium carbonate, calcium borate, nanosized silica, light-weight ceramic, EVA redispersable rubber powder and the like.The building wall coating has the advantages of being high in breaking strength and compressive strength, has the effects of heat preservation and sound insulation, is low in heat conductivity coefficient and good in energy-saving effect, and has the good waterproof effect.The obtained wall material is portable, low in cost, free of pollution and environmentally friendly, energy consumption and the production cost are greatly reduced, and the material can be widely applied to building construction.
Owner:韩旭霞
Ceramic jade white glaze and glaze application method
The invention discloses ceramic jade white glaze. The comprises the following ingredients, by weight, 48-52 parts of albite, 5-8 parts of calcium carbonate, 7-9 parts of kaolin, 33-36 parts of quartz, 5-7 parts of barium carbonate, 0.4-0.6 part of calcium borate, 1-3 parts of strontium carbonate, 0.5-1.5 parts of bentonite and 0.01-0.02 part of methyl cellulose. Glazing modes comprise glaze soaking and glaze spraying twice. The sintering curve comprises four stages of 1 DEG C-300 DEG C, 300 DEG C-600 DEG C, 600 DEG C -1000 DEG C and 600 DEG C-1330 DEG C. The ceramic jade white glaze has a low cost and a wide adaptability, the saying is changed that Dehua ceramic whiteware only depends on good porcelain clay in history, a ceramic product made from the provided ceramic jade white glaze and through the glaze application method is oily and fleshy like Hetian jade and is ivory, and a layer of brightness appears on the glaze surface. Especially, when thickening is carried out during glaze application of a green body especially, the glaze flows vertically when high temperature sintering and when the glaze fluid flows and deposits, the Hetian jade texture is strong and the lightspot is shown.
Owner:FUJIAN DEHUA JINGHUA CERAMIC CO LTD
Nano borate engine oil
InactiveCN105400577AReasonable formula designReasonable designAdditivesN dimethylformamideAntioxidant
The invention discloses nano borate engine oil and belongs to the technical field of engine oil formulas. The nano borate engine oil is prepared from API II type base oil, a molybdenised abrasion-resistant and energy-saving agent, alkylphenol, cetyl calcium borate, nano cerium borate, a defoaming agent, a clearing agent, N,N-dimethylformamide, an ashless dispersing agent, hydroxy stearate, dimeticone, sulfonate, a succinimide dispersing agent, talcum powder, amine antioxidant, ethylene glycol butyl ether, a viscosity index improver, molybdenum disulfide, graphite powder, mono-succinimide, sorbitan monooleate, petroleum ether, chloroform, friction modifier sulfurized isobutylene, dodecenylsuccinic acid and phosphite ester. The nano borate engine oil is good in dynamic property, capable of saving energy, environmentally friendly, low in friction coefficient, small in ash content and long in service life.
Owner:烟台狮王石化工业有限公司
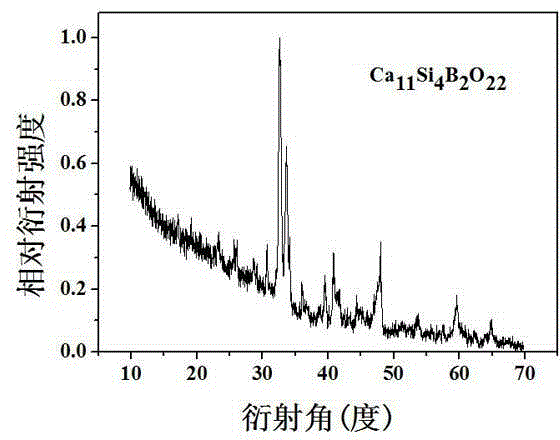
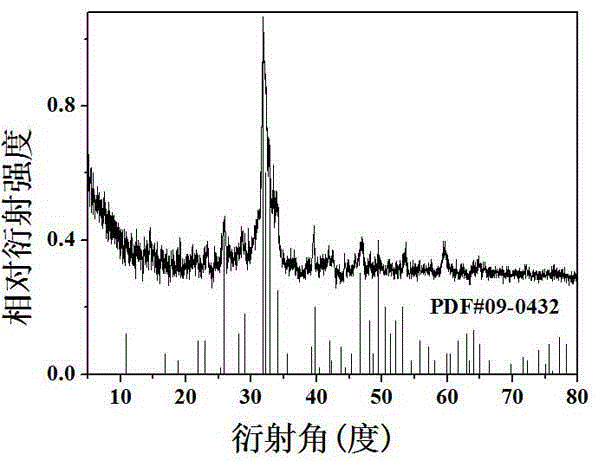
Calcium borate silicate biological material as well as preparation and application thereof
ActiveCN103819182AGood biocompatibilityNo side effectsImpression capsDentistry preparationsChemical synthesisApatite
The invention relates to a calcium borate silicate biological material as well as preparation method and application thereof. Pure-phase calcium borate silicate Ca11Si4B2O22 powder is synthesized by a solid-phase synthesis method or a wet chemical synthesis method; the calcium borate silicate powder is pressed and moulded to obtain a calcium borate silicate ceramic block; the calcium borate silicate powder or the calcium borate silicate ceramic block is soaked in a simulated body fluid (SBF) for certain time and mineralized; after a while, a bone-like apatite layer can deposit on the surface of the powder or the ceramic block; researches find that the thickness of mineralized hydroxyapatite increases along with the increase of the soaking time. The calcium borate silicate powder or the ceramic material prepared through the preparation method has good biological performance, is a biological and medical material with very high potential, and can be used as a bone tissue repairing and filling material and a tooth repairing material.
Owner:安徽(淮北)新型煤化工合成材料基地管理委员会科技服务中心
Ceramic product with self-cleaning glaze and making method thereof
The invention provides a ceramic product with self-cleaning glaze. The ceramic product comprises a tire material and the glaze, wherein the tire material comprises kaolin, quartz, potassium feldspar,clay and talc; the glaze comprises ground glaze and cover glaze, wherein the ground glaze comprises the following raw materials: potassium feldspar, nano zinc oxide, calcium oxide, burning talc, zirconium silicate, wollastonite, kaolin, bone ash, calcium borate and frit; the cover glaze comprises the following raw materials: potassium feldspar, kaolin, wollastonite, zinc oxide, calcium oxide, aluminum oxide, magnesium oxide, zirconium oxide and calcium borate. A making method of the ceramic product with the self-cleaning glaze comprises the following steps: (1) respectively mixing the tire material and the glaze, adding water, wet-milling with a ball mill and sieving to obtain tire material slurry and glaze slurry; (2) making a green body with the tire material slurry for biscuit firing; (3) applying the ground glaze; (4) applying the cover glaze; (4) firing. The ceramic product made of the making method provided by the invention is fine and bright in glazed surface and can effectivelyprevent the accumulation of dirt and the formation of black spots.
Owner:福建省德化龙辉陶瓷有限公司
Fireproof fired clay hollow brick and preparation method thereof
InactiveCN105967696AUniform Flame RetardancyUniform fire resistanceCeramic materials productionClaywaresPolyvinyl alcoholSlag
The invention discloses a fireproof fired clay hollow brick and a preparation method thereof. The fireproof fired clay hollow brick is prepared from the following raw materials (by weight): 15-25 parts of Benshan green mud, 17-29 parts of picrite, 7-14 parts of high aluminum fine powder, 9-18 parts of fused magnesite, 13-21 parts of periclase, 10-15 parts of high boron calcium borate, 16-24 parts of petroleum coke slag, 11-19 parts of kyanite ore tailings, 5-10 parts of sodium metaantimonate, 18-26 parts of brown fused alumina slag, 14-22 parts of silica fume, 32-46 parts of attapulgite, 17-23 parts of polyvinyl alcohol, 8-14 parts of magnesium silicate, 12-18 parts of barite, 10-15 parts of chromite slag, 13-21 parts of sawdust, 19-33 parts of fly ash and a proper amount of water. The prepared fired clay hollow brick has both excellent flame retardancy and fire resistance, long-term operation temperature of the fired clay hollow brick is also greatly raised. Actual operation temperature can reach 1350 DEG C and above.
Owner:安徽宏发节能设备有限公司
Preparation method of calcium borate/graphene oxide nano composite lubricant
ActiveCN105969478AOvercome time-consuming disadvantagesSimple preparation processAdditivesCalcium borateWear testing
The invention provides a preparation method of a calcium borate / graphene oxide nano composite lubricant, relating to the technical field of lubricant preparation. The calcium borate nanoparticles are loaded onto the graphene oxide surface by liquid-phase ultrasonic mixing peeling, thereby synthesizing the calcium borate / graphene oxide nano composite lubricant. The time consumption for preparation is only 7-12 hours, thereby lowering the energy consumption, lowering the cost and enhancing the loading capacity of the calcium borate on graphene oxide sheets and the lubricating property in the base oil. When a friction test is carried out on a four-ball friction wear testing machine of which the load is 147N and the rotation speed is 1200 r / min, the friction factor of the composite lubricant prepared by the method in the base lubricating oil can be lowered by 30-50% at the maximum.
Owner:YANGZHOU UNIV
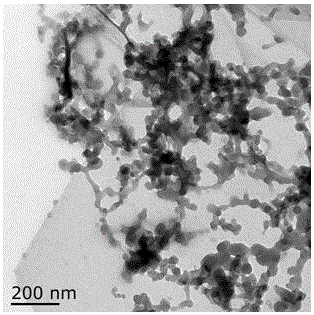
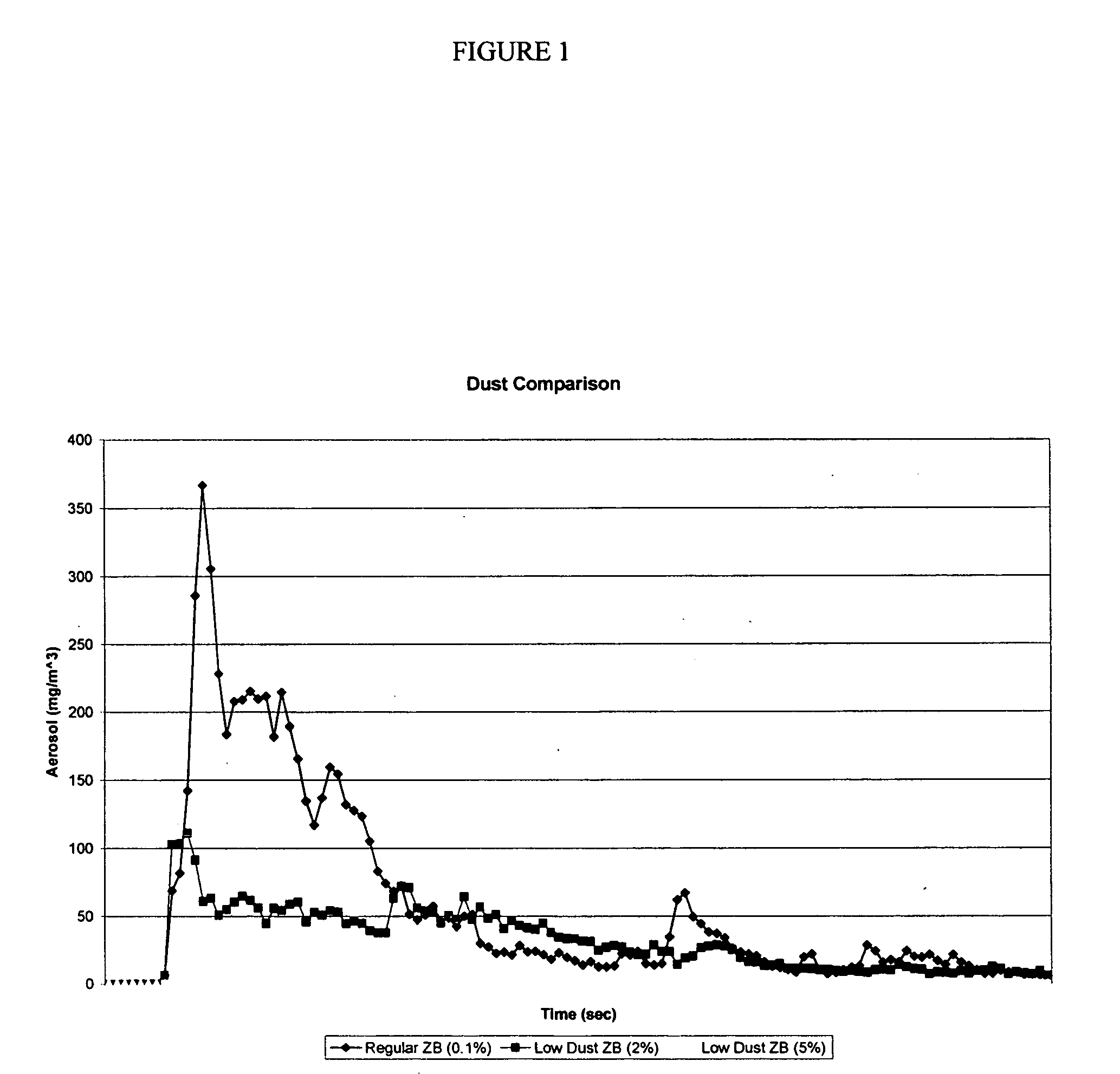
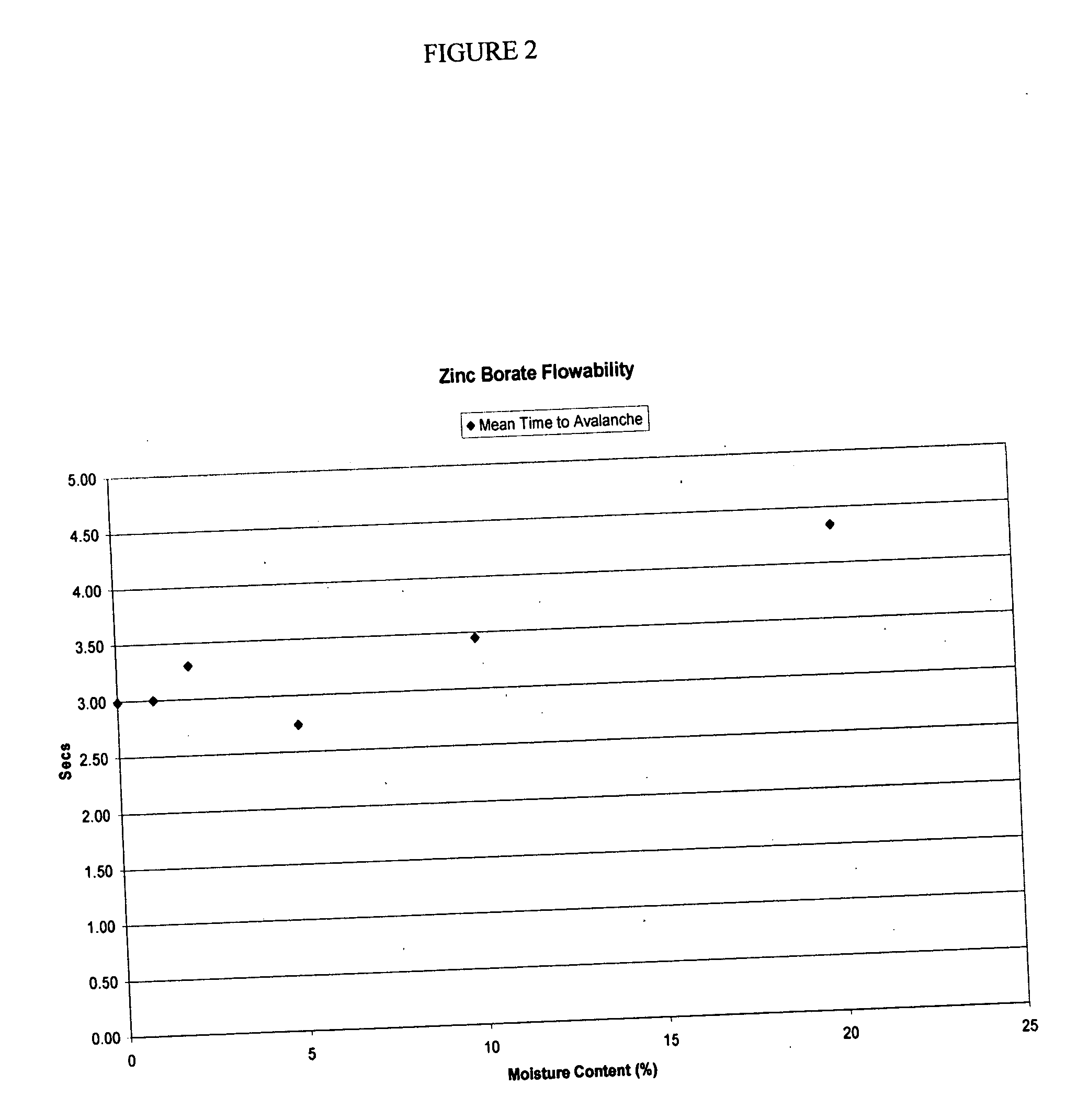
Low dust preservative powders for lignocellulosic composites
ActiveUS20070001337A1Reduce dustEvenly distributedOther chemical processesWood working apparatusCalcium borateCellulose
The manufacture of zinc borate and calcium borate powders in a water slurry and drying those powders in a controlled manner such as to leave a desired residual of moisture content uniformly dispersed throughout the product produces a low dust, flowable material. This low dust material results in environmental and economic benefits to users of these preservative borates. The preferred amount of residual moisture is from 2 to 10 percent.
Owner:NISUS CORP
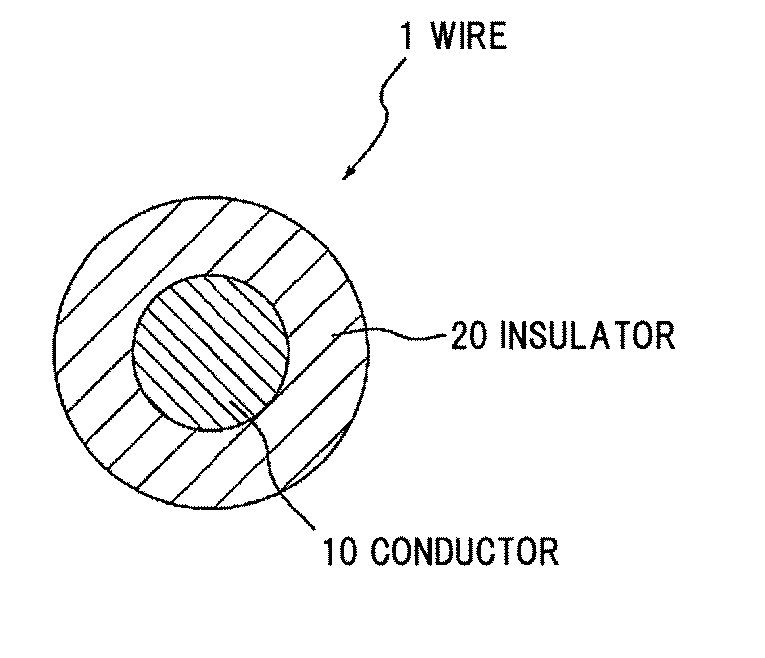
Halogen-free flame-retardant resin composition, wire and cable
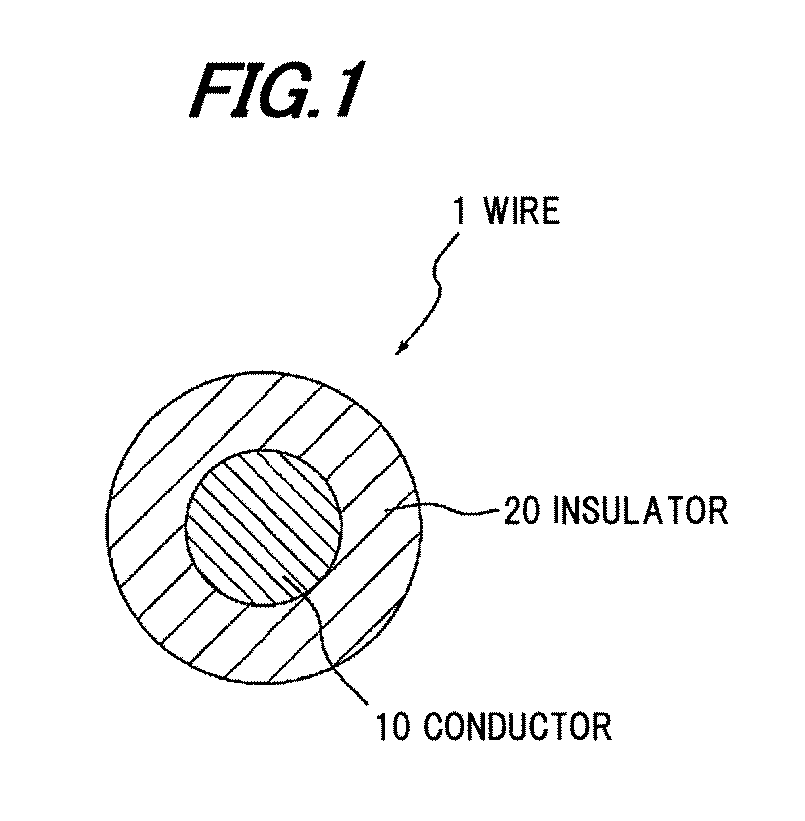
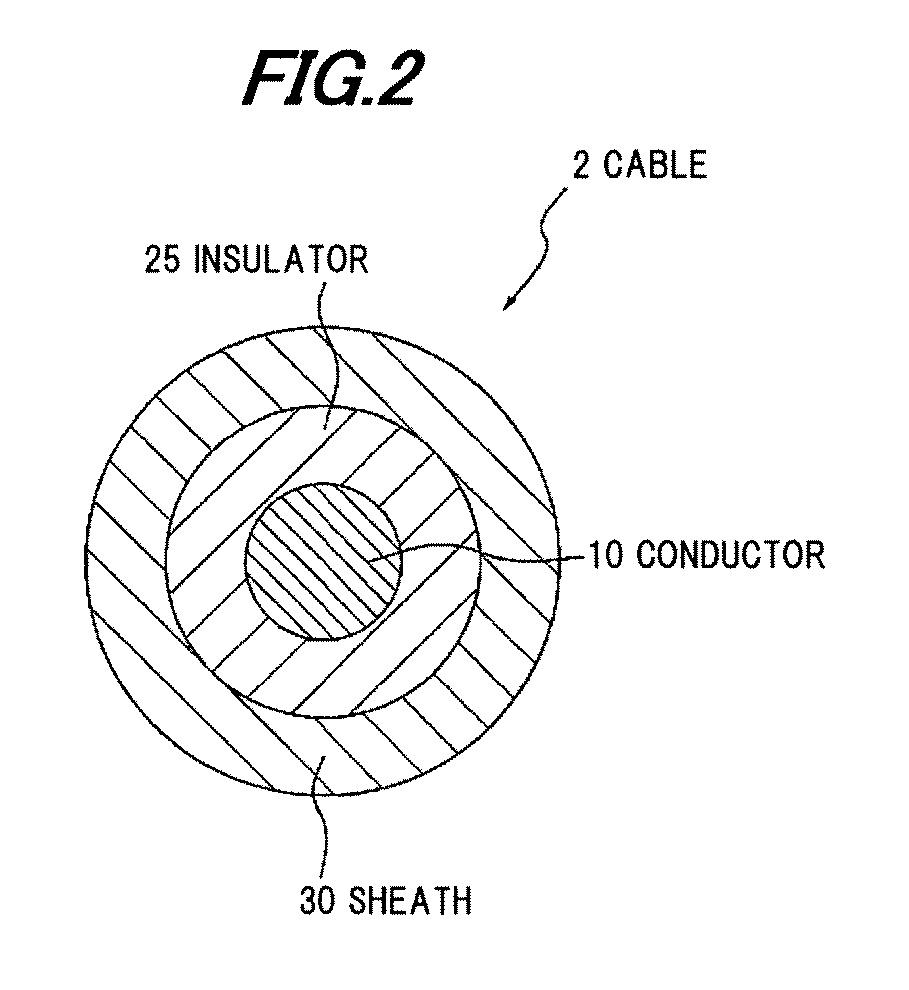
InactiveUS20120003473A1Plastic/resin/waxes insulatorsSynthetic resin layered productsCalcium boratePolyolefin
Owner:HITACHI CABLE
Compound calcium borate, calcium borate optical crystal, preparation method of calcium borate optical crystal, and uses of calcium borate optical crystal
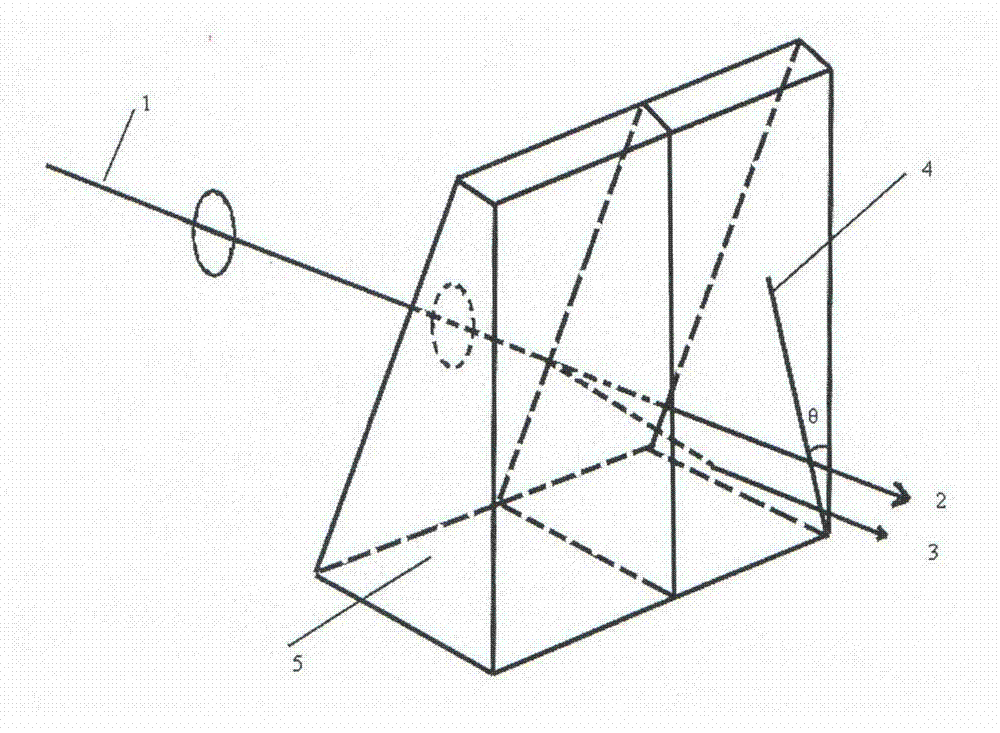
InactiveCN104775159ACut wellEasy to polishPolycrystalline material growthBy pulling from meltSpace groupBeam splitting
The present invention relates to a compound calcium borate, a calcium borate optical crystal, a preparation method of the calcium borate optical crystal, and uses of the calcium borate optical crystal. According to the present invention, the chemical formula of the compound is Ca2B2O5, the molecular weight is 181.78, the space group is P2[1] / c, the crystal cell parameters comprise that a is 3.5582(5)angstrom, b is 6.3503(8)angstrom, c is 19.299(3)angstrom, beta is 92.386 DEG, V is 435.70(10)angstrom<3>, and Z is 4, and the solid state reaction method is used to synthesize; the chemical formula of the calcium borate optical crystal is Ca2B2O5, the calcium borate optical crystal belongs to the monoclinic system, the space group is P2[1] / c, the crystal cell parameters comprise that a is 3.5582 (5)angstrom, b is 6.3503(8)angstrom, c is 19.299(3)angstrom, beta is 92.386 DEG, V is 435.70(10)angstrom<3>, and Z is 4; the transmission range of the calcium borate optical crystal is 180-3000 nm, the birefringence is moderate, and the crystal has characteristics of easy cutting, polishing and preserve, stability in air, deliquescence resistance, and insolubility in water; and the calcium borate optical crystal can be used for producing polarizing prisms, phase delay devices, electro-optical modulators or beam splitting polarizers and other devices, wherein the devices utilize the refractive index characteristic of the crystal, and the important applications are provided in the fields of optics and communication.
Owner:XINJIANG TECHN INST OF PHYSICS & CHEM CHINESE ACAD OF SCI
Frit suitable for color development as well as colored vitreous enamel product and preparation method
The invention relates to a frit suitable for color development as well as a colored vitreous enamel product and a preparation method. The frit suitable for color development comprises the following components in parts by weight: 20-35 parts of feldspar, 10-45 parts of quartz, 0.5-5 parts of Suzhou clay, 2-6 parts of calcium borate, 1-7 parts of aluminium hydroxide, 12-32 parts of light calcium carbonate, 1-8 parts of barium carbonate, and 1-8 parts of borax. The preparation method for the colored vitreous enamel product comprises the following steps: (1), preparing enamel slurry; (2), enameling the product; (3), sintering. The colored vitreous enamel product comprises an inner enamel layer and an outer enamel layer, wherein the ceramic carcass is high-end ceramic, the inner enamel layer is a transparent frit enamel layer, and the outer enamel layer is a colored enamel layer made from the frit suitable for color development. The frit suitable for color development is appropriate in frit expansion factor, relatively wide in sintering range, bright and flat in enamel surface, and can be used for various colored decoration of various high-end ceramic products. The preparation method of the colored vitreous enamel product and the colored vitreous enamel product can be used for producing various high-end enamel products for hue decoration.
Owner:山东省淄博华洋陶瓷有限责任公司
Composite of food-grade compound calcium sulfonate-base lubricating grease and preparation method thereof
InactiveCN102899134AImprove high temperature resistanceImprove shear stabilityThickenersCalciteStearic acid
The invention relates to a composite of food-grade compound calcium sulfonate-base lubricating grease and a preparation method of the composite. The preparation method comprises the following steps of: feeding mixture of 40-60% synthesized calcium sulfonate with high base number, water and ethanol into a reaction kettle, stirring and heating to 60-80DEG C, adding 0.5-2% of acetic acid to convert calcium carbonate with a calcite structure, adding 1-5% of calcium hydroxide and 2-5% of stearic acid under 90-100 DEG C after 1h of constant temperature, carrying out saponification reaction for 120min, rising temperature after saponification, adding 1-5% of calcium borate after 1h of 130-140 DEG C constant temperature, rising the temperature to 180-200DEG C keeping constant temperature for 5-10min, cooling the temperature to 100-110DEG C, adding 0.2-1.5% of antioxygen, homogenizing, adjusting thickness by adding base oil, degassing in a filtering way, and filling to form finished products. The dropping point of the composite provided by the invention is more than 300DEG C, the 0.1-milliom times shear difference value is within 20, and the PD (potential difference) value is not less than 400kg.
Owner:CHINA PETROLEUM & CHEM CORP
Preparation method of cobalt boracylate
InactiveCN101643480AImprove anti-static performanceFine grainGroup 3/13 element organic compoundsAcetic acidCalcium borate
The invention provides a preparation method of cobalt boracylate, belonging to the field of the preparation of composite material accelerating agent. The preparation method comprises the following steps: mixing neodecanoic acid, isooctyl acid, glacial acetic acid, dimethylbenzene and cobalt hydroxide, carrying out acid-base neutralization reaction, and obtaining an intermediate product after the neutralization reaction is finished; adding tributyl borate into the obtained intermediate product and carrying out boracylate reaction, adding calcium borate as additive simultaneously, and obtaininga reaction product, namely, cobalt boracylate after the boracylate reaction is finished. In the preparation method, the neodecanoic acid, the isooctyl acid, the lower fatty acid glacial acetic acid and the dimethylbenzene are matched and carry out the acid-base neutralization reaction together with the cobalt hydroxide to generate the intermediate product; the reaction velocity can be improved andthe forming of the intermediate product is accelerated due to the effects of the glacial acetic acid and the dimethylbenzene; in addition, in the final boracylate reaction, as the calcium borate as the additive is added together with the tributyl borate, the reaction time is effectively shortened, and the insoluble substance performance and the anti-static property of the cobalt boracylate whichis prepared finally are improved.
Owner:大连爱柏斯化工股份有限公司
Technology for treatment of fluorine-boron-containing wastewater during Ceftezole acid preparation
InactiveCN102963999AReduce fluorine contentIncrease valueMultistage water/sewage treatmentNature of treatment waterAlkali freePotassium
The invention discloses a technology for treatment of fluorine-boron-containing wastewater during Ceftezole acid preparation. According to the technology, boric acid and fluoboric acid are remove from wastewater respectively by a two-stage neutralization reaction, so that the fluorine content of the industrial wastewater is decreased to 5mg / l, the boron content is reduced to 5mg / l, and the pH of the wastewater is 6-7, thus meeting the national wastewater discharge standards. The technology has the advantages of simple operation, small investment, and large by-product value. Calcium borate can be used for alkali-free glass fiber production, and potassium fluoborate can be used as a catalyst for polypropylene synthesis.
Owner:江苏德峰药业有限公司
Composite calcium-based lubricating grease and preparation method thereof
ActiveCN101921651AImproved Surface Hardening PropertiesSimple arrangementAdditivesCalcium borateCalcium biphosphate
The invention discloses composite calcium-based lubricating grease and a preparation method thereof. The composite calcium-based lubricating grease consists of lubricating grease base oil, a composite calcium-based thickener and a surfactant in a mass part ratio of (225-398):(40.8-72.4):(1-4), wherein the lubricating grease base oil is at least one of vacuum cut 2 base oil, vacuum cut 3 base oil and vacuum cut 4 base oil; the composite calcium-based thickener is a thickener consisting of calcium C12 to C24 fatty acid soap and a calcium auxiliary acid salt in a mass part ratio of (25-44.4):(15.8-28); and the calcium auxiliary acid salt is at least one of calcium C1 to C22 fatty acid salt, calcium borate and calcium phosphate. The preparation method of the invention comprises the steps of saponification, recombination and thickening and esterification. In the composite calcium-based lubricating grease, the surfactant and the soap molecules produce a synergistic effect, so the arrangement mode and base oil immobilizing capacity of the soap molecules are improved and the surface hardening performance of the composite calcium-based lubricating grease is improved.
Owner:CHINA NAT OFFSHORE OIL CORP +2
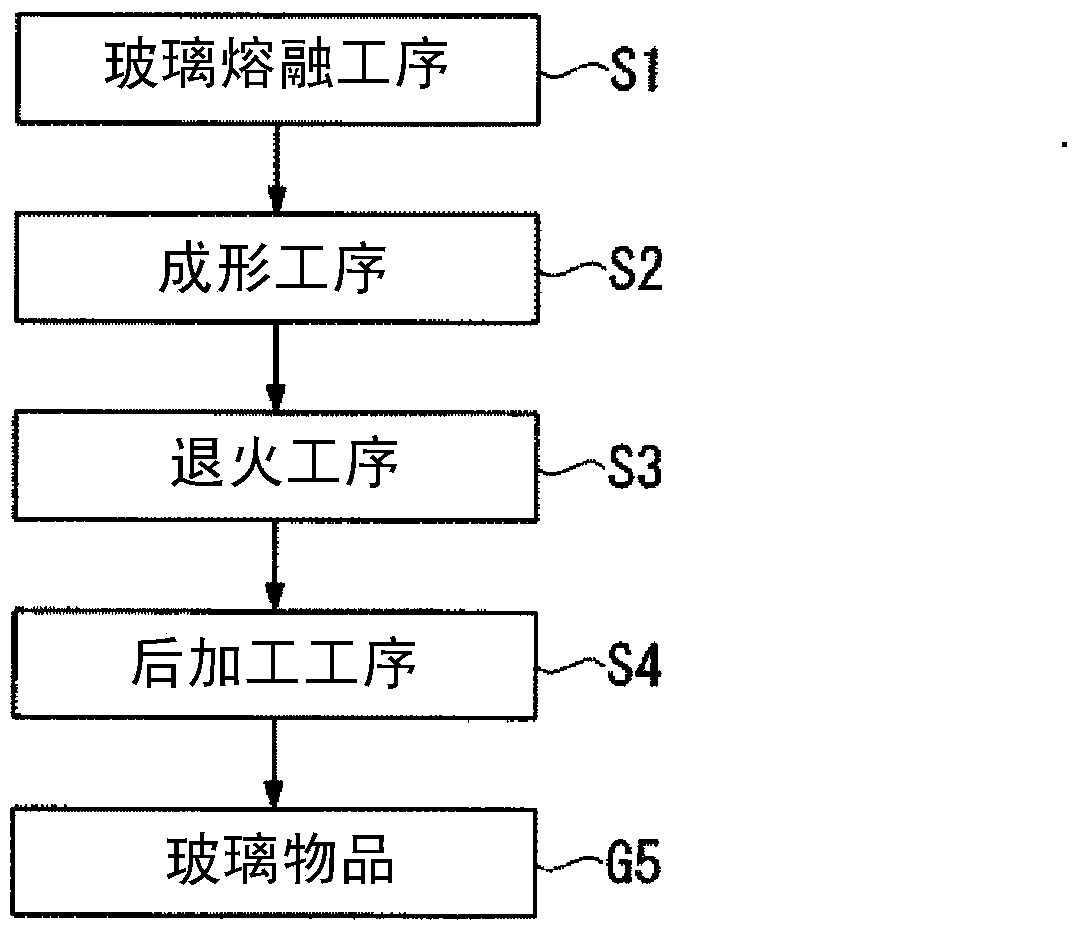
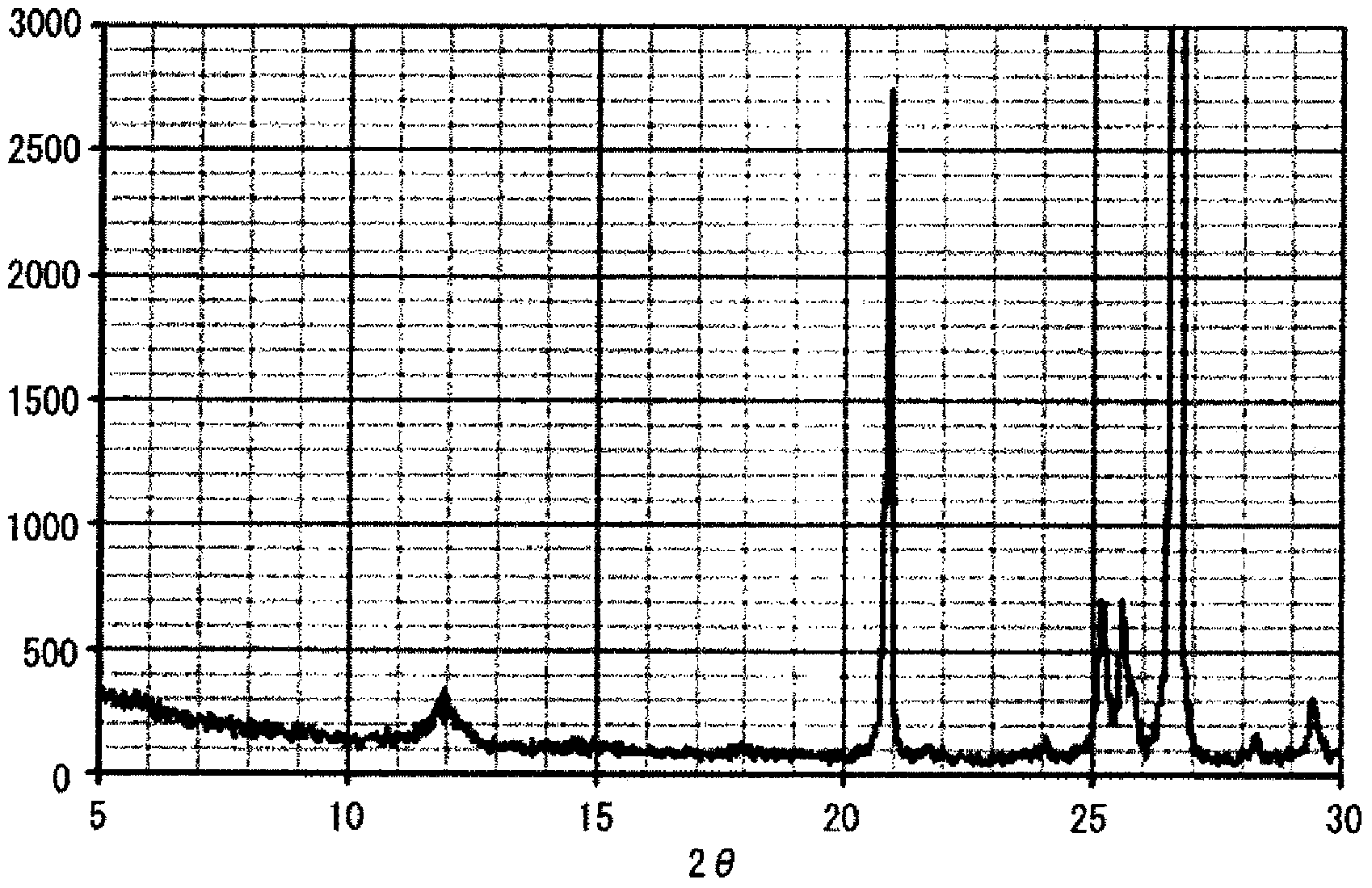
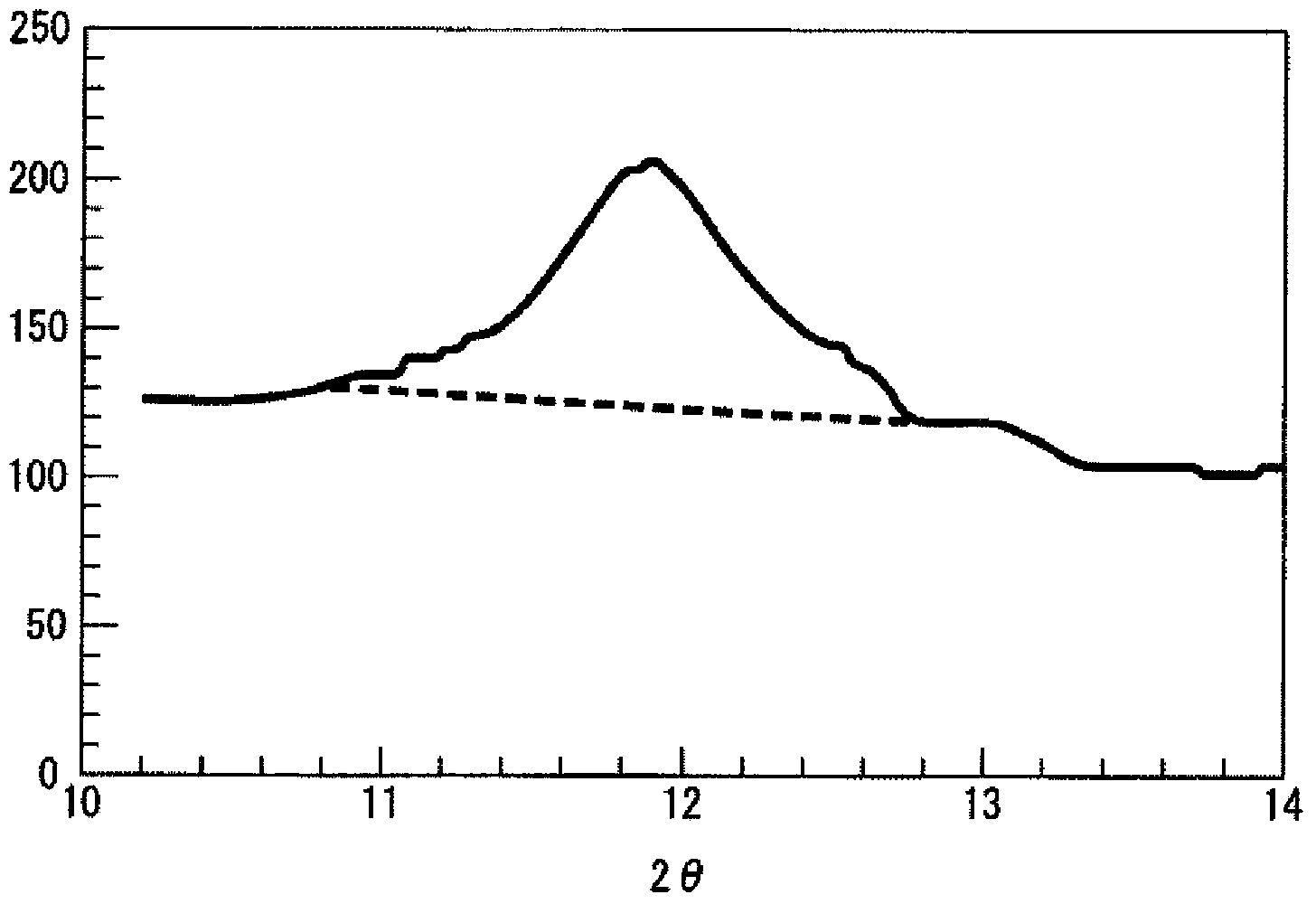
Granules and method for producing same, production method for molten glass, and production method for glass article
ActiveCN103547541AReduce contentHigh strengthCharging furnaceGlass shaping apparatusAlkali freeAlkali metal oxide
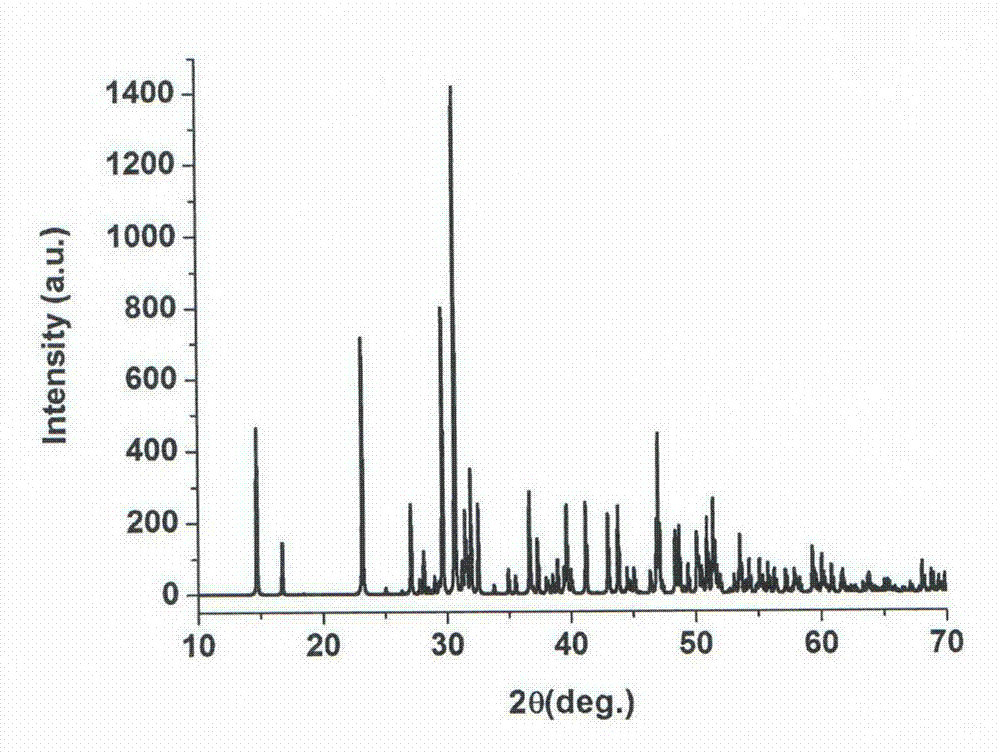
The present invention relates to granules, which are a glass raw material mixture for the production of alkali-free glass containing substantially no alkali metal oxides, wherein the glass structure of glass obtained from the granules is, in mol% on an oxide basis, 60-75 mol% SiO2, 5-15 mol% Al2O3, 1-9 mol% B2O3, 0-15 mol% MgO, 0-20 mol% CaO, 0-12 mol% SrO, 0-21 mol% BaO, and more than 0 mol% for the total of CaO, SrO and BaO; and for which in an X-ray diffraction spectrum obtained by means of a CuKa X-ray powder diffraction, when the diffraction peak area of quartz (100) in which 2Theta is 19.85-21.71 degrees is taken as 1, the total of the relative value of the diffraction peak area of strontium borate hydrate in which 2Theta is 10.81-13.01 degrees, the relative value of the diffraction peak area of calcium borate hydrate in which 2Theta is 11.11-13.49 degrees, and the relative value of the diffraction peak area of barium borate hydrate in which 2Theta is in the range of 10.91-13.27 degrees, is at least 0.005. The present invention also relates to a production method for the granules, a method for producing molten glass using the granules, and a method for producing a glass article using the production method for molten glass.
Owner:AGC INC
Preparation method of high-voltage-resistant solid polymer electrolyte
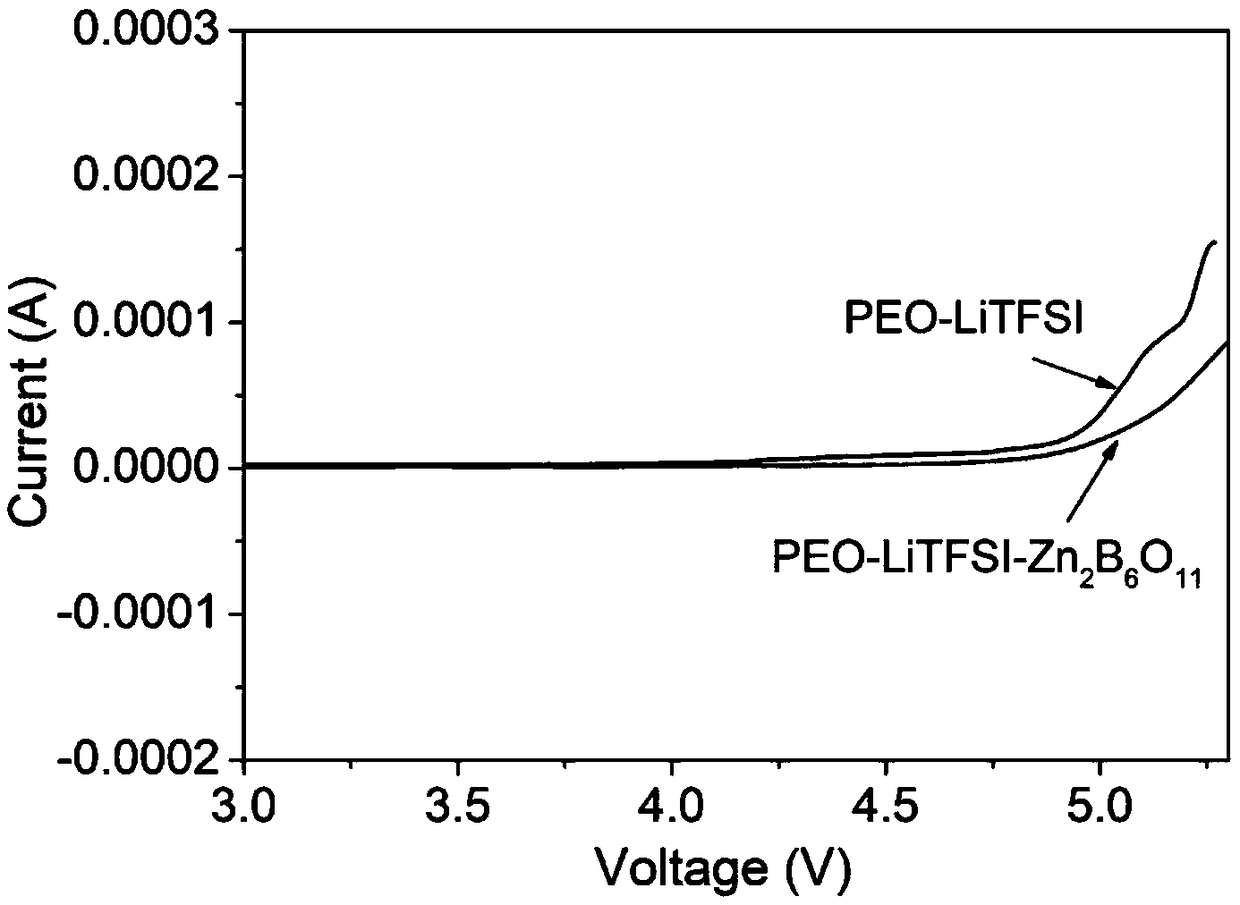
ActiveCN109301317AImprove high pressure performanceIncrease energy densitySolid electrolytesLi-accumulatorsPolymer scienceNanowire
A preparation method of a high-voltage-resistant solid polymer electrolyte includes the steps of: 1) according to certain ratio, dissolving a polymer substrate, lithium salt, and an inorganic additivein anhydrous acetonitrile and stirring the mixture at room temperature to obtain a uniform solution, wherein the polymer substrate is polyoxyethylene, the lithium salt is lithium bistrifluoromethylsulfonimide or lithium perchlorate; the inorganic additive is nano-wires or nano-particles of zinc borate, aluminum borate, sodium tetraborate, barium metaborate or calcium borate, the mass ratio of thepolyoxyethylene to the lithium salt EO : Li<+> is 10-20:1, and the mass of the inorganic additive is not more than 20% of the total mass of the polymer substrate and lithium salt; 2) pouring the solution into a polytetrafluoroethylene mold to volatilize the solution until completely dried, thus preparing the solid polymer electrolyte. The method improves the high-voltage resistance of the solid polymer electrolyte, so that the product fits a high-voltage ternary cathode material and energy density and safety of an all-solid-state battery are improved.
Owner:ZHEJIANG UNIV OF TECH
Matte ancient porcelain and preparation technology thereof
ActiveCN107619256AImprove bindingNot easy to fall offCeramic materials productionClaywaresCalcium borateSodium Bentonite
The invention discloses matte ancient porcelain and a preparation technology thereof. The matte ancient porcelain comprises a green body and matte glaze covering the green body. The matte glaze is prepared from the following raw materials in parts by weight: 10 to 15 parts of kaolin, 12 to 16 parts of zhangzhou clay, 15 to 25 parts of quartz, 30 to 40 parts of matte frit, 8 to 10 parts of bentonite, 1 to 3 parts of aluminum oxide, 0.5 to 0.8 part of calcium borate and 0.2 to 0.5 part of medical stone powder; the matte frit is prepared from the following chemical components in percentage by mass: 46 to 53 percent of SiO2, 12 to 16 percent of Al2O3, 8 to 14 percent of Na2O, 6 to 10 percent of K2O, 5 to 8 percent of MgO, 0.5 to 1.2 percent of PbO, 1 to 2 percent of Y2O3, 1.8 to 2.5 percent ofSnCl2, 3 to 5 percent of CaCO3 and 2 to 3 percent of MnO2.
Owner:德化县万宝古建陶瓷有限公司
Nickel-based cell anode, its preparation method and nickel-based cell by using anode
ActiveCN103647054AEvenly distributedImprove performanceAlkaline accumulator electrodesNickel accumulatorsCalcium borateSalt calcium
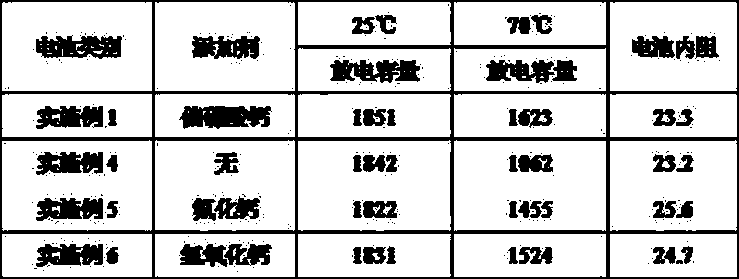
The invention discloses a nickel-based cell anode, its preparation method and a nickel-based cell by using the anode. A technical scheme main point is characterized in that the nickel-based cell anode comprises an anode active material, the anode active material contains nickel hydroxide and soluble calcium salt calcium metaborate or calcium borate calcium borate, wherein the content of soluble calcium salt calcium metaborate or calcium borate accounts for 0.01-5% of weight of nickel hydroxide. The invention also discloses a preparation method of the nickel-based cell anode and an alkalescence nickel-based secondary cell by using the nickel-based cell anode. According to the invention, calcium ion uniform distribution and introduction of beneficial anion in the soluble calcium salt can effectively improve the comprehensive performance of the nickel-based cell, the nickel-based cell internal resistance is reduced, the high temperature performance is increased, and the cycle performance is improved.
Owner:河南省恒明新能源有限公司
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com