Method and device for preparing ethylphenylhydrazine hydrochloride by pipelines
A technology of o-ethylphenylhydrazine and ethylphenylhydrazine, which is applied in the field of compound preparation, can solve problems such as inability to quickly achieve uniform mixing at the molecular scale, increase side reactions, and instability of diazonium salts, so as to overcome local uneven concentration , reduced residence time, simple post-processing effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
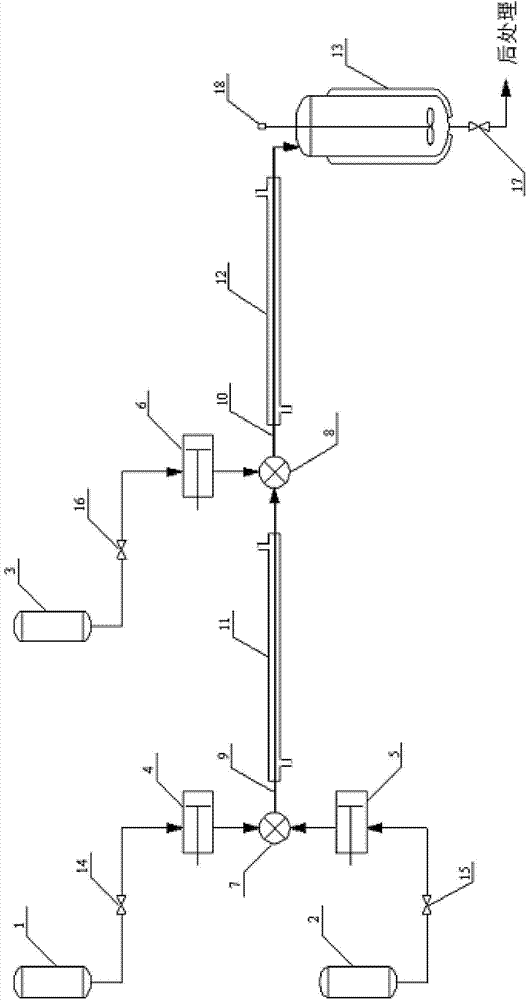
[0024] The structure of the reaction device is as figure 1 , the first tubular reactor: a single tube with a length of 1 m and a diameter of 1 mm; the second tubular reactor: a single tube with a length of 30 m and a diameter of 1 mm.
[0025] The operation steps are as follows:
[0026] Prepare o-ethylaniline hydrochloric acid aqueous solution: o-ethylaniline (1212g, 10mol), 37wt% concentrated hydrochloric acid (2470g, containing HCl 25mol), water 1725g;
[0027] Prepare sodium nitrite aqueous solution: sodium nitrite (690g, 10mol), water 1380g;
[0028] Preparation of sodium sulfite aqueous solution: sodium sulfite (3150g, 25mol), water 12600g;
[0029] The first tubular reactor 9 is preheated and kept at 80° C. by jacket a11, and the o-ethylaniline hydrochloric acid aqueous solution and the sodium nitrite aqueous solution are respectively stored in the first storage container 1 and the second storage container 2, and Continuously input into the first mixer 7 through the ...
Embodiment 2
[0031] The structure of the reaction device is as figure 1 , The second tubular reactor: the length of the tube is 10 m, and the diameter of the tube is 4 mm.
[0032] 37wt% concentrated hydrochloric acid consumption is 3450g (wherein containing HCl 35mol) in o-ethylaniline hydrochloric acid aqueous solution; Sodium nitrite consumption is 794g (11.5mol) in sodium nitrite aqueous solution; The molar flow ratio of o-ethylaniline and sodium nitrite is 1: 1.15; the residence time in the first tubular reactor 9 is 1s; in the reactor 13, the reaction is kept at 70°C for 3 hours;
[0033]Other operations were the same as in Example 1, and 950 g of o-ethylphenylhydrazine hydrochloride was finally obtained with a yield of 55% and a melting point of 180.3-181.5°C.
Embodiment 3
[0035] The structure of the reaction device is as figure 1 , The second tubular reactor: the length of the tube is 1 m, and the diameter of the tube is 10 mm.
[0036] 37wt% concentrated hydrochloric acid consumption is 3450g (wherein containing HCl 35mol) in o-ethylaniline hydrochloric acid aqueous solution; Sodium nitrite consumption is 794g (11.5mol) in sodium nitrite aqueous solution; The molar flow ratio of o-ethylaniline and sodium nitrite is 1: 1.15; the residence time in the first tubular reactor 9 is 1s; the reaction is kept at 95°C for 1.5h in the reactor 13;
[0037] Other operations were the same as in Example 1, and 967 g of o-ethylphenylhydrazine hydrochloride was finally obtained, with a yield of 56% and a melting point of 179.8-181.5°C.
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com