Buzane-based azine structure compound based on meta-position linkage and preparation method and application thereof
A compound, hydrazine technology, applied in the field of analysis and detection, can solve the problems of inability to realize the application of red fluorescent probes, and achieve the effect of excellent aggregation-induced luminescence characteristics
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0066] Embodiment 1: the synthesis of cyano salicylaldehyde azine (CN-SAA)
[0067]
[0068] Reflux 0.5g of p-cyanobenzene salicylaldehyde and hydrazine hydrate under 25ml of ethanol for 4h (add acetic acid as a catalyst), remove the ethanol under reduced pressure after cooling, extract, and separate the organic phase with a column after drying to obtain 0.52g of a light yellow solid. It is the CN-SAA structure. MALDI-TOF (m / z): [M+]calcd.C 28 h 18 N 4 o 2 , 442.143; found, 443.15.Anal Calc.for C 28 h 18 N 4 o 2 : C, 76.01; H, 4.10; N, 12.66; O, 7.23. Found: C, 75.78; H, 4.04; N, 12.33; O, 7.05.
Embodiment 2
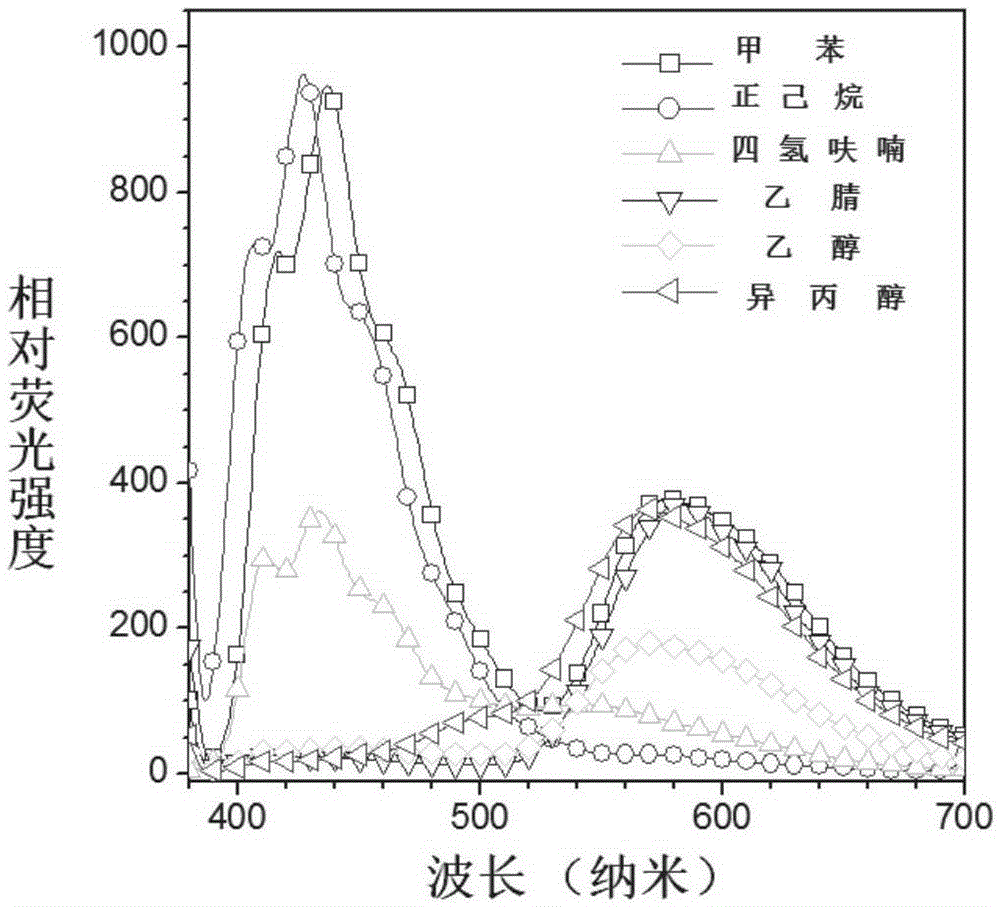
[0069] Example 2: Study on the ESIPT and AIE properties of CN-SAA
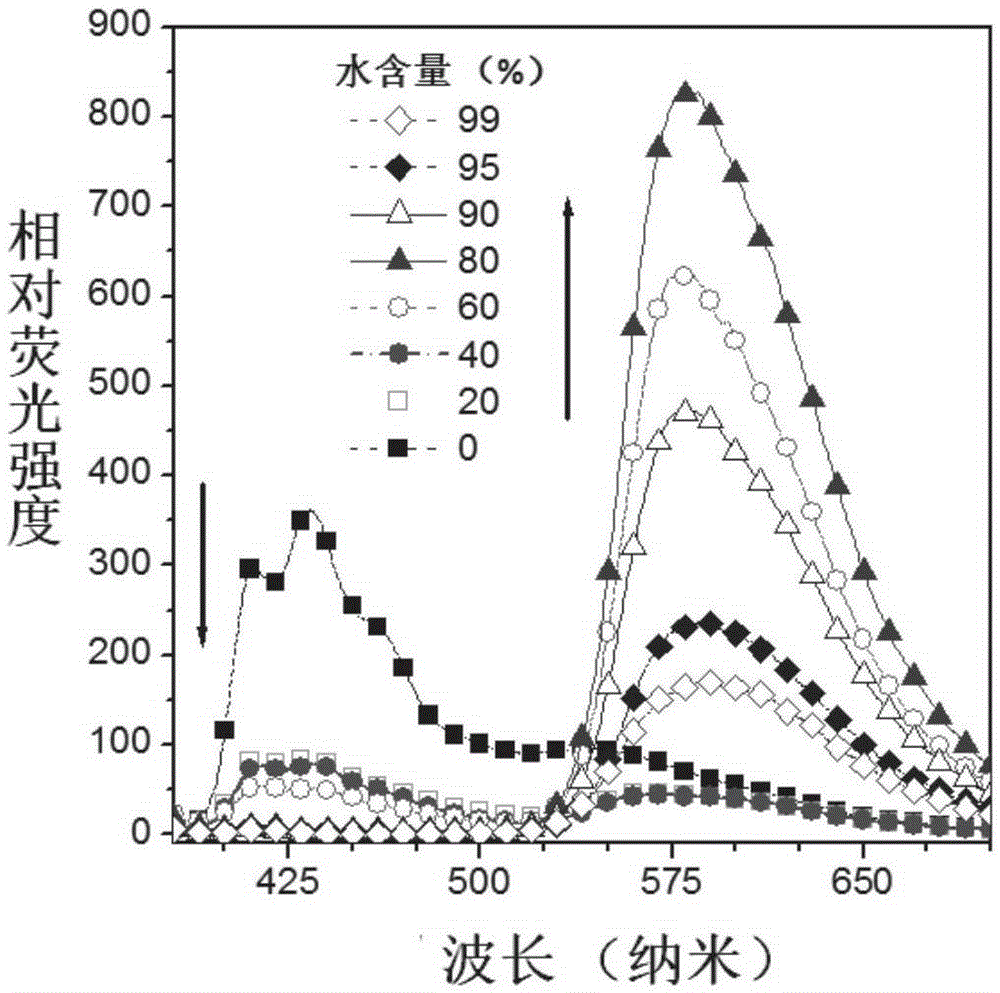
[0070] Such as Figure 1A It is the fluorescence spectrum of CN-SAA under different polarities. With the change of polarity, the ratio of alcohol emission (near 426nm) and ketone emission (near 579nm) of CN-SAA changes obviously, which is a typical ESIPT emission . For ESIPT molecules, the aggregation state is mostly ketone emission, so we detected the aggregation luminescence phenomenon at this place. Such as Figure 1B As shown, a certain proportion of water was continuously added to the THF solvent of CN-SAA (dissolved single molecular state), and CN-SAA slowly aggregated into nanoparticles due to solubility problems, and the fluorescence intensity of the alcohol form gradually decreased, and the intensity of the ketone form increased. The proportion of those increased with the increase of water content, indicating that CN-SAA has AIE properties. The ESIPT and AIE properties suggest that CN-SAA has the p...
Embodiment 3
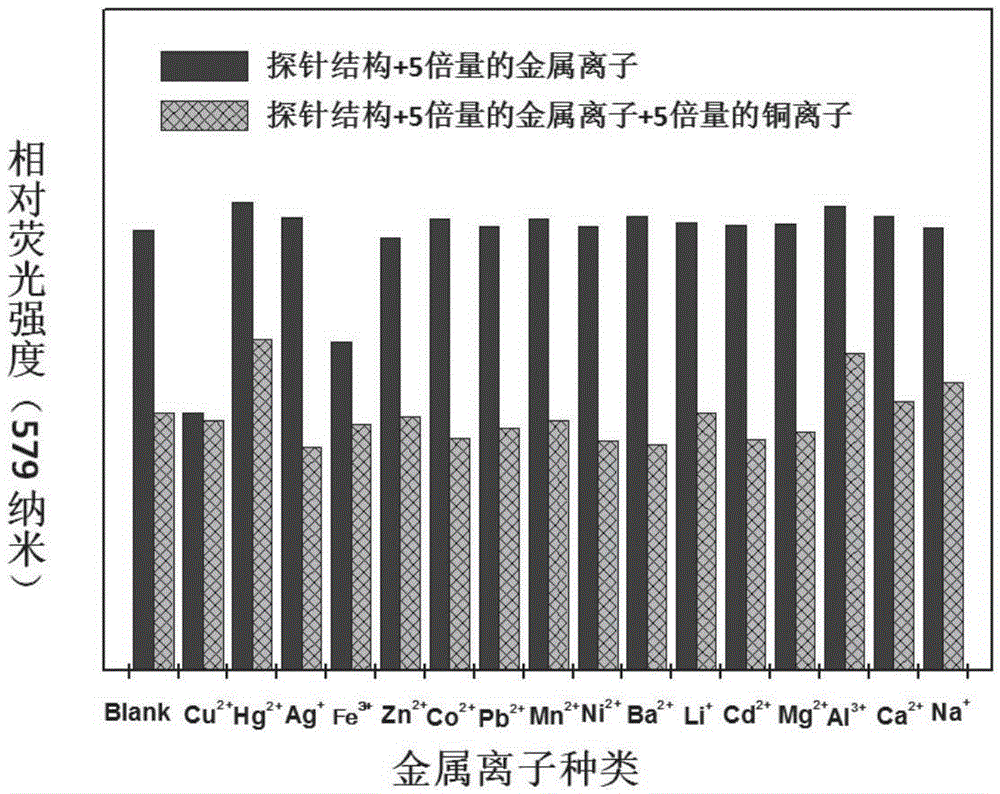
[0071] Example 3: Research on the responsiveness of CN-SAA to metal ions
[0072] Such as Figure 2A with 2B Shown are the experimental results of the selection of different metal ions by CN-SAA in aqueous solution. Through comparison, it is found that the fluorescent probe has a specific response to copper ions, and its fluorescence intensity around 579nm is significantly reduced, and there is a certain linear relationship. ; Through the interference experiment results, it can be seen that the detection of copper ions has little influence on the presence of other ions.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com