Trans-octadecaborane derivative as well as preparation method and application thereof
A technology of octadecaborane and its derivatives, which is applied in the field of trans-octadecaborane derivatives and its preparation to achieve excellent aggregation-induced luminescent properties
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
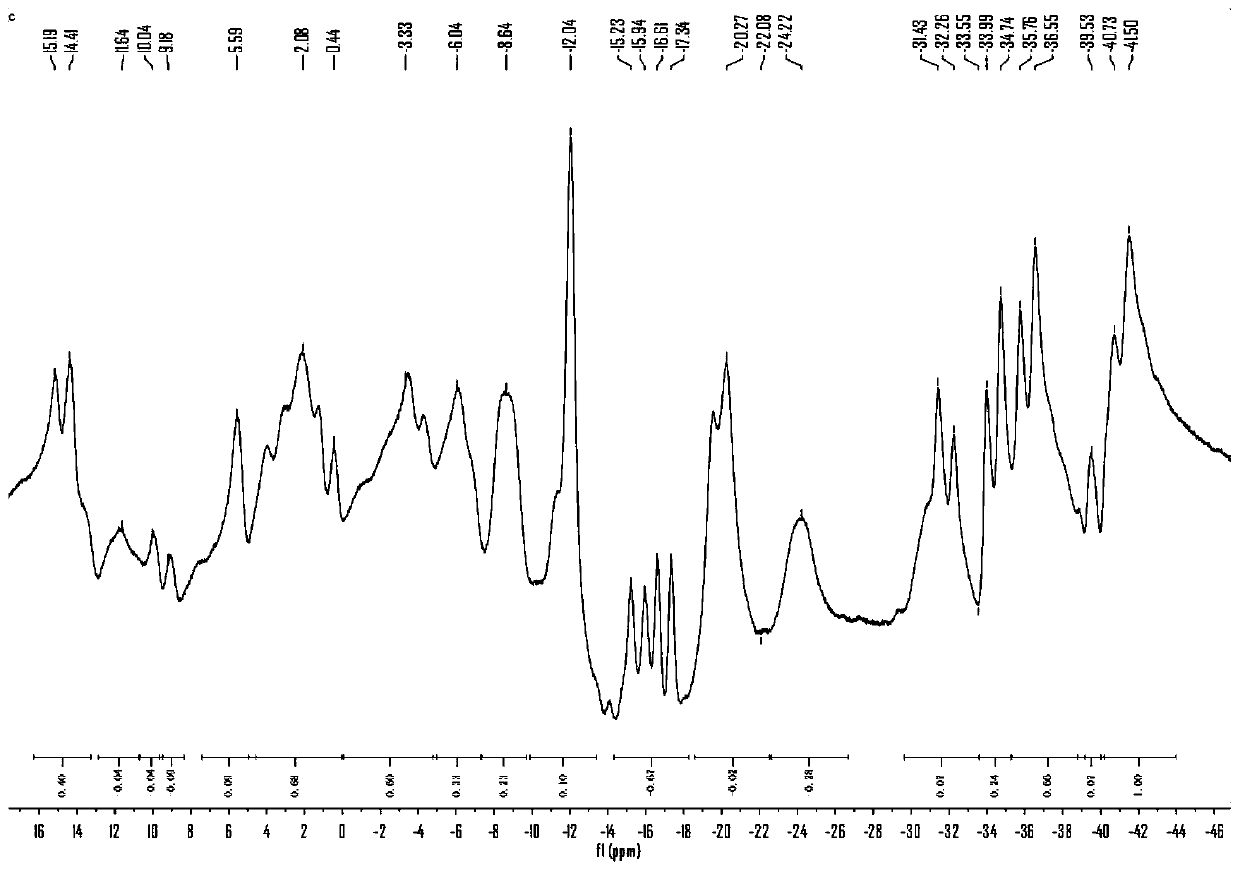
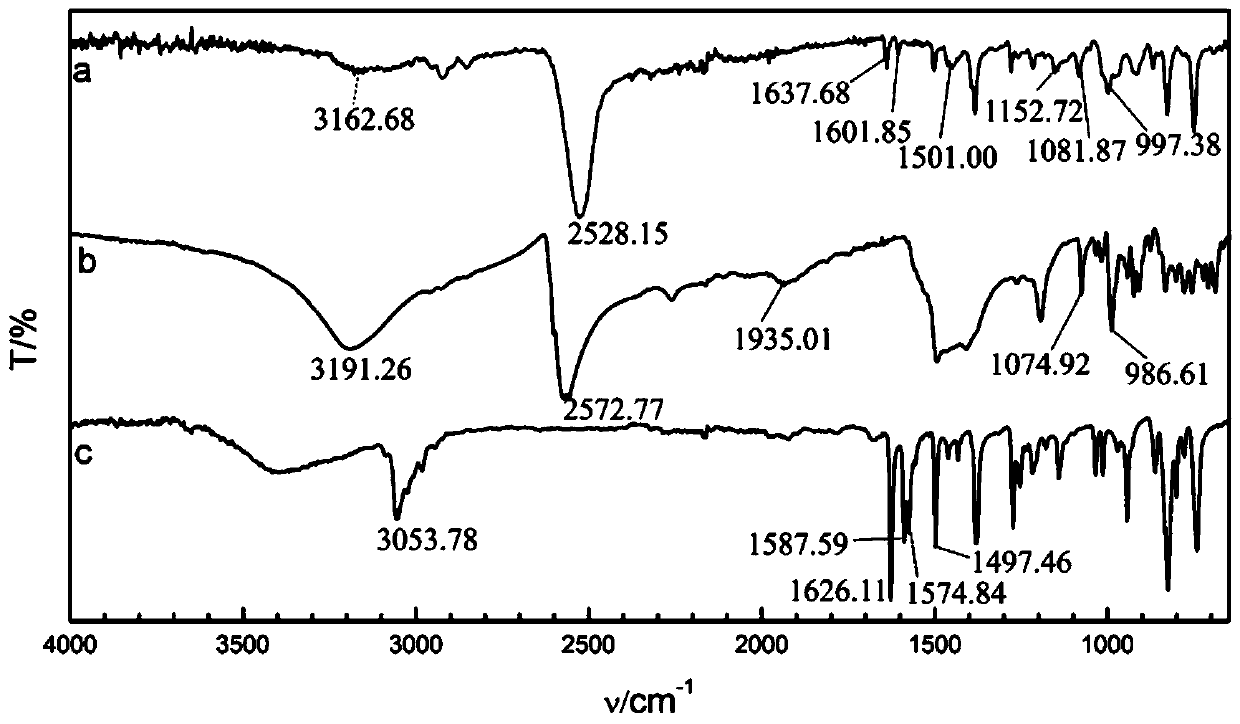
[0029] The preparation method of the above trans-octadecaborane derivative. The preparation route is:
[0030]
[0031] The specific preparation process includes the following steps: 1) The isoquinoline and trans-octadecaborane are reacted in a solvent to obtain isoquinoline-substituted anti-B 18 H 22 Crude compound; 2) anti-B substituted with isoquinoline 18 H 22 The crude compound is obtained after purification.
Embodiment 1
[0033] This embodiment provides a preparation method of trans-octadecaborane derivative, which specifically includes the following steps:
[0034] 1) Add isoquinoline (0.505ml, 4.297mmol) dropwise to 20ml of dry benzene solution, and stir on a magnetic stirrer for 10min to disperse evenly, record it as solution A; then add the weighed trans 18 Anti-B 18 H 22 (200mg, 0.923mmol) into a 100ml round bottom flask, and add 60ml of dry benzene solution, and stir on a magnetic stirrer for about 15 minutes to anti-B 18 H 22 Completely dissolve, record it as solution B; add solution A slowly to solution B, the solution immediately turns yellow, and with the increase of solution A, the yellow color continues to deepen to orange-yellow, mark the mixture of A and B as C Solution: Heat and stir C solution under the protection of nitrogen atmosphere to make the benzene solution reflux. During this process, a yellow precipitate is formed, reflux for 10 hours and stop heating.
[0035] 2) After the...
Embodiment 2
[0042] This embodiment provides a method for preparing trans-octadecaborane derivatives. The difference from Example 1 is that: isoquinoline and trans-octadecaborane are directly put into a dry benzene solution in sequence, Heat and stir the C solution under protection to make the benzene solution reflux. Other conditions are the same as in Example 1. Finally, the reaction time of step 1) is prolonged by at least 2 times.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com