Preparation and application of a class of trifluoromethyl-modified platinum complexes
A platinum complex, trifluoromethyl technology, applied in the field of phosphorescent materials, to achieve the effect of excellent aggregation-induced luminescence properties
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
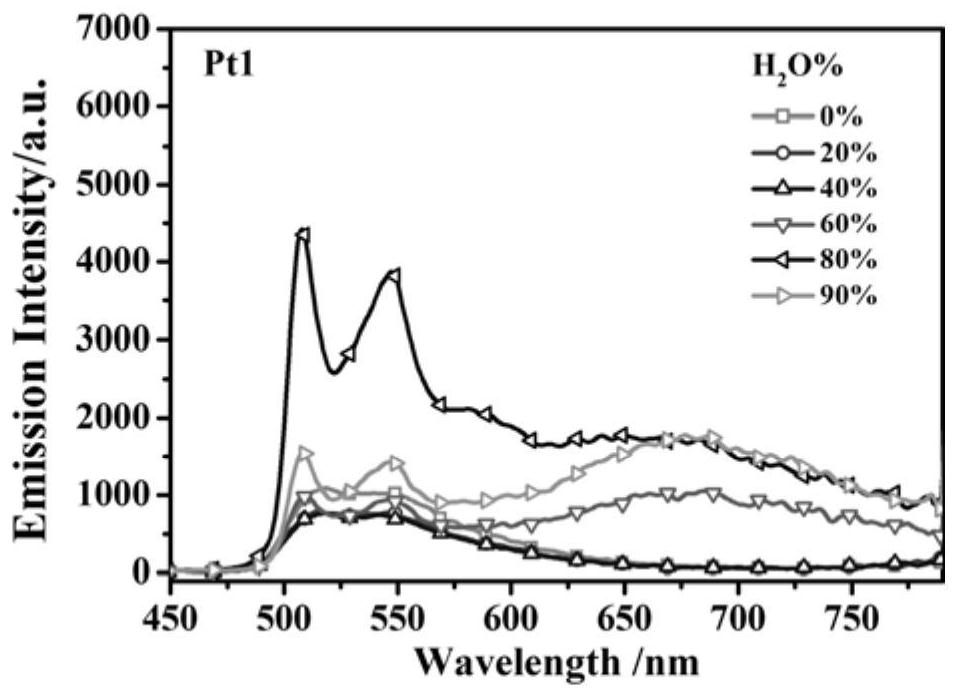
[0032] The synthesis of embodiment 1 complex Pt1
[0033] (1) Synthesis of cyclometal ligands:
[0034] In the air, add 1.0mmol of 2-bromo-4-trifluoromethylpyridine, phenylboronic acid (1.5equiv.), potassium carbonate (2.0equiv.), palladium acetate (1.5%equiv.) , and then add 8 mL of ethanol-water mixed solution with a volume ratio of 3:1, and carry out Suzuki cross-coupling reaction under magnetic stirring at 80 ° C. Track the reaction process by thin-layer chromatography. After the reaction is complete, add 20 mL of saturated saline, Extract with dichloromethane, combine the organic phases, concentrate under reduced pressure, and separate by column chromatography to obtain the ring metal ligand.
[0035] (2) Synthesis of neutral platinum complexes:
[0036] Add cyclometal ligands, 1.2 equivalents of potassium tetrachloroplatinate, and 8 mL of ethylene glycol monoethyl ether / water (3:1, v / v) mixed solution into a 25 mL round-bottomed two-neck flask, under nitrogen protectio...
Embodiment 2
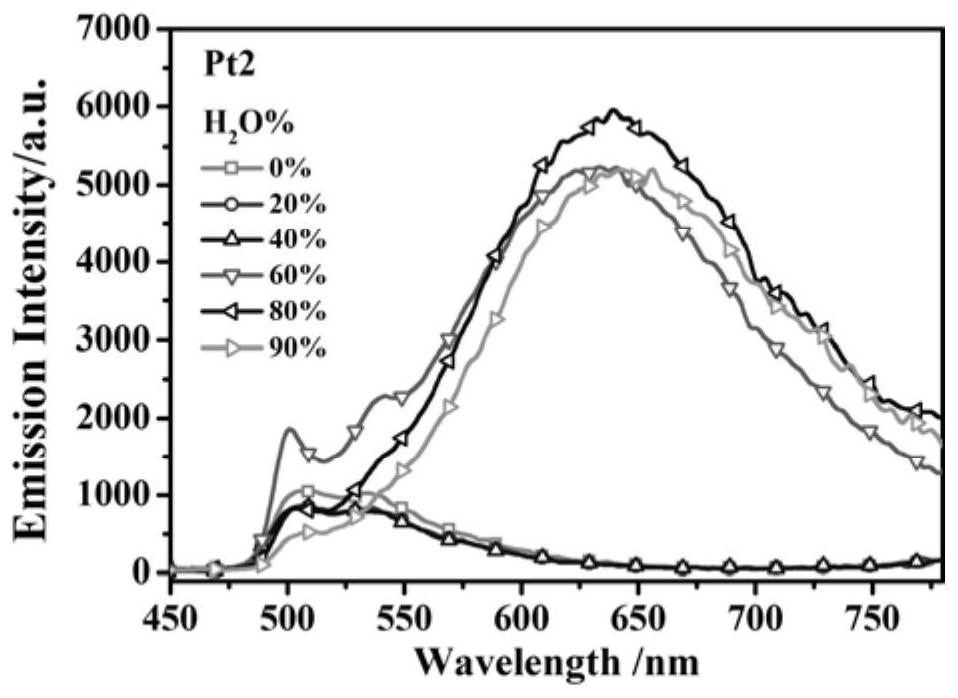
[0037]The synthesis of embodiment 2 complex Pt2
[0038] The preparation method of Example 2 is the same as that of Example 1, except that the 2-bromopyridine derivative used in the synthesis of the ring metal ligand in Example 2 is 2-bromo-5-trifluoromethylpyridine.
[0039] The yield of Pt2 is 64%, and the structural characterization data are as follows: 1 H NMR (400MHz, CDCl 3 )δ9.30(s,1H),7.99(d,J=8.2Hz,1H),7.68(d,J=8.4Hz,1H),7.63(d,J=7.6Hz,1H),7.47(d, J=7.6Hz,1H),7.24(s,1H),7.12(t,J=7.4Hz,1H),5.49(s,1H),2.03(d,J=2.4Hz,6H).HRMS(EI, m / z) theoretical value: 517.0697[M+H] + , measured value: 517.0703[M+H] + .
Embodiment 3
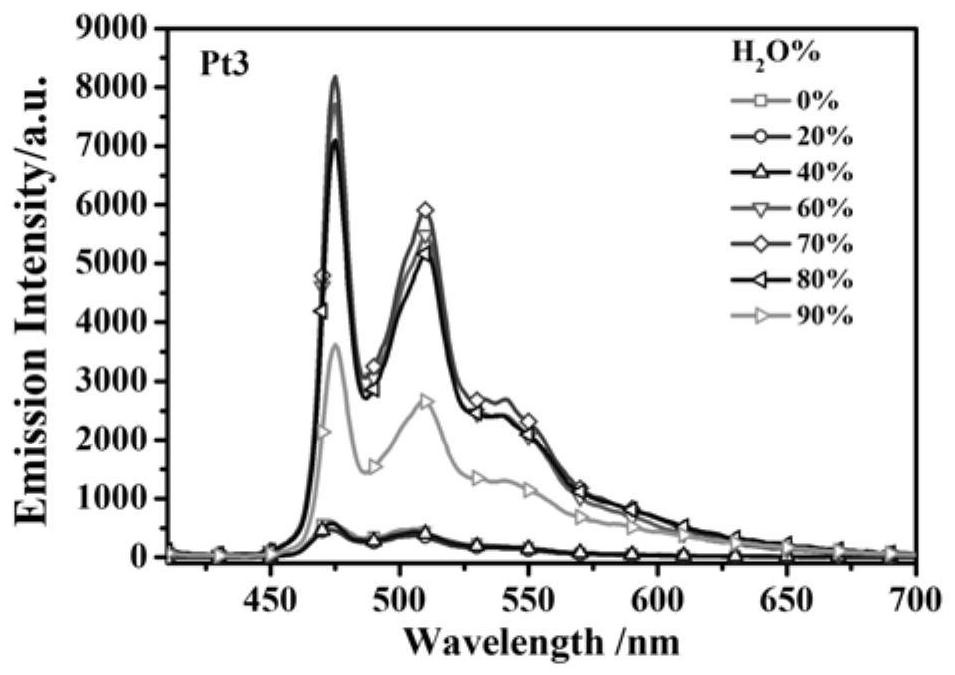
[0040] The synthesis of embodiment 3 complex Pt3
[0041] (1) Synthesis of cyclometal ligands:
[0042] In air, add 1.0 mmol of 2-bromopyridine, 3-trifluoromethylphenylboronic acid (1.5 equiv.), potassium carbonate (2.0 equiv.), and palladium acetate (1.5% equiv.) to a round-bottomed two-necked flask successively, Then add 8 mL of ethanol-water mixed solution with a volume ratio of 3:1, and carry out Suzuki cross-coupling reaction with magnetic stirring at 80 ° C. Track the reaction process by thin-layer chromatography. After the reaction is complete, add 20 mL of saturated saline, and use Extract with dichloromethane, combine the organic phases, concentrate under reduced pressure, and separate by column chromatography to obtain the ring metal ligand.
[0043] (2) Synthesis of platinum complexes:
[0044] Add cyclometallic ligands, 1.2 equivalents of potassium tetrachloroplatinate and 8 mL of ethylene glycol monoethyl ether / water (3:1, v / v) mixed solution into a 25 mL round-...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com