Amphiphilic ethylene derivative and preparation method
An ethylene derivative and amphiphilic technology, which is applied in the fields of ether preparation, ester reaction preparation of ether, chemical instruments and methods, etc., can solve problems such as difficulty in dihedral angle, and achieve the effects of simple structure, convenient synthesis, and excellent aggregation-induced luminescence performance.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] Synthesis of α,α'-Dimethoxytetraphenylethylene
[0036] Dibromostilbene (2.028g, 6mmol), α-methoxyphenylboronic acid (4.56g, 30mmol), potassium carbonate (4.416g, 30mmol), palladium tetrakistriphenylphosphine (693mg, 0.6mmol) In a 250mL round bottom bottle; under nitrogen protection, add 100mL toluene, 2mL water, 2mL ethanol, stir until dissolved, raise the reaction temperature to 110°C, and continue the reaction for 18h; after the reaction, extract with dichloromethane (3×50mL) , the organic phases were combined; the solvent was removed after drying with anhydrous magnesium sulfate; column chromatography separation was performed using n-hexane / dichloromethane (90 / 10) as the mobile phase to obtain a white solid with a yield of 73%.
Embodiment 2
[0038] Synthesis of α,α'-Dihydroxytetraphenylethylene
[0039] Put α,α'-dimethoxytetraphenylethylene (0.392g, 1.0mmol) in a 50mL reactor; add 10mL of dry dichloromethane under nitrogen protection, stir until dissolved; cool to -65°C, stir 15min; add 4.0mL of boron tribromide in dichloromethane solution (1.0mol / L), take out the reactor, and put it at room temperature; stir at room temperature for 12h; take 100mL of deionized water and place it in a 250mL round bottom bottle; Bubbling for 10 min; pour the liquid in the reactor into a round-bottomed bottle under nitrogen protection, and stir for 1 h; extract with dichloromethane (3×50 mL), combine the organic phases; dry with anhydrous magnesium sulfate and remove the solvent to obtain a white Solid, 90% yield.
Embodiment 3
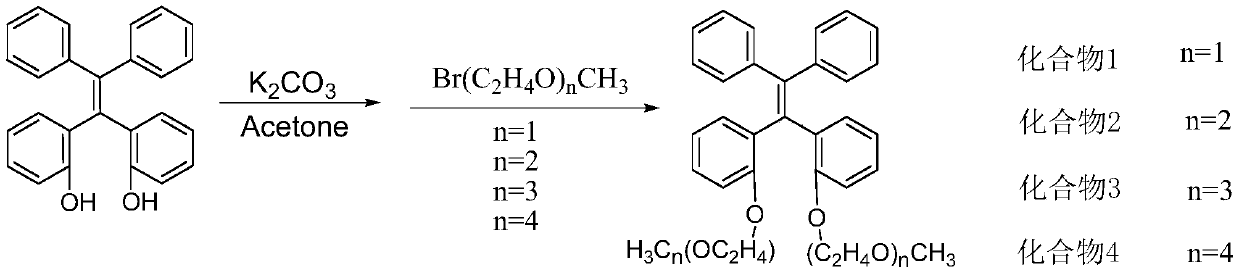
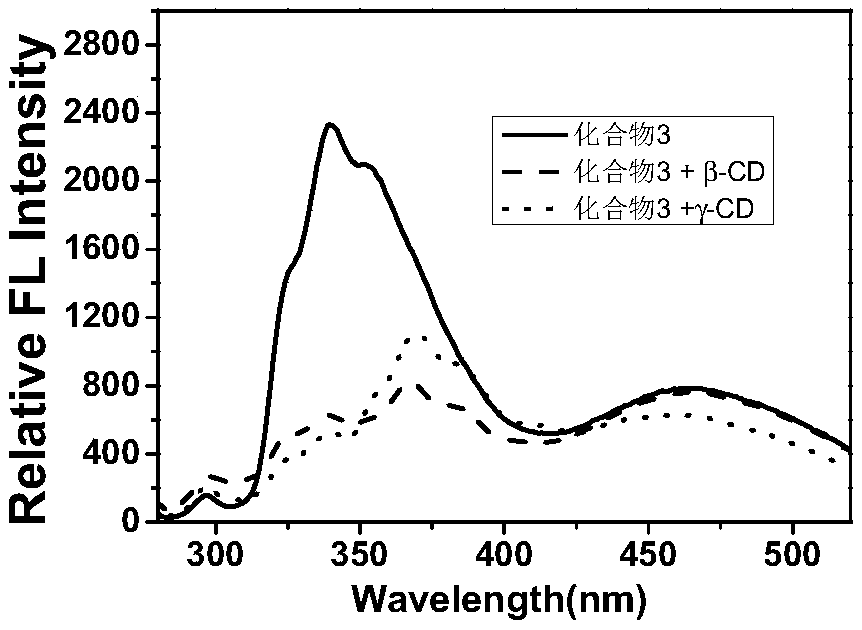
[0042] Such as figure 1 Synthesis of shown compound 1 (n=1 alkoxy chain substituted tetraphenylethylene)
[0043] Put α,α'-dihydroxytetraphenylethylene (0.364g, 1mmol) and potassium carbonate (1.38g, 10mmol) in a 150mL round bottom bottle; add 50mL of dry acetone under nitrogen protection, stir until dissolved; add 2 -Bromoethyl methyl ether (0.556g, 4mmol); warm up to 60°C, react for 12h; extract with ethyl acetate (3×50mL), combine the organic phases; use anhydrous magnesium sulfate to remove the solvent after drying; use n-hexane / ethyl acetate (80 / 20) was used as the mobile phase for column chromatography to obtain a light yellow viscous liquid with a yield of 80%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com