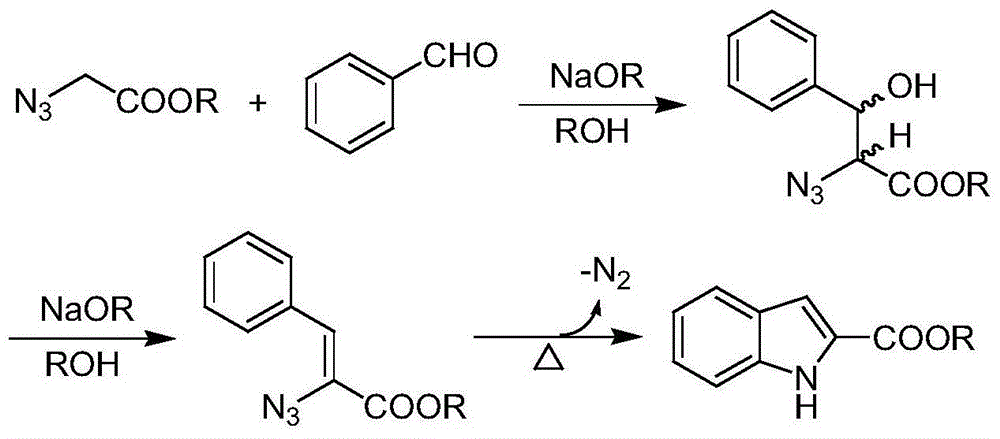
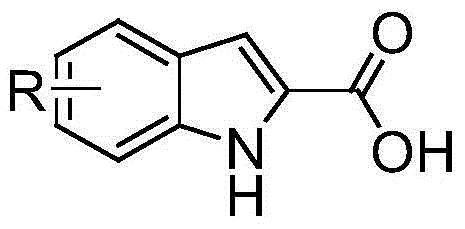
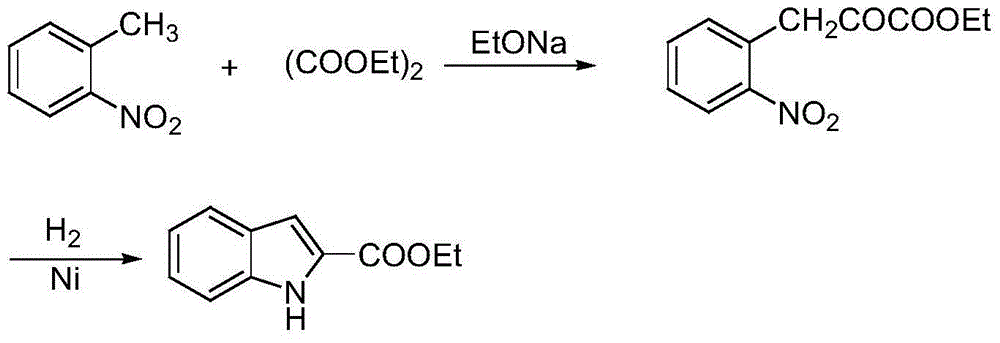Synthetic method of substituted indol-2-formic acid
A synthesis method and indole technology, which is applied in the synthesis of important intermediates of medicine and pesticides, and in the field of synthesis of substituted indole-2-carboxylic acid, can solve the problem of waste gas, waste water, waste, unfavorable industrial application, complex process, etc. problems, to achieve the effects of environmental friendliness, significant social and economic benefits, and easy availability of raw materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0035] Example 1. Synthesis of indole-2-carboxylic acid
[0036] Using ethanol as the solvent, heat 108 g of phenylhydrazine, 1 ml of acetic acid and 127 g of ethyl pyruvate in a mixed solvent of 1175 ml of ethanol and 235 ml of water, and reflux at a temperature of 80°C. Monitor the completion of the reaction for 3 hours. After the solvent, it was recrystallized with a 70% ethanol aqueous solution, filtered, and dried to obtain 177 g of light yellow crystal phenylhydrazine pyruvic acid phenylhydrazone with a yield of 86%.
[0037] Step 2: Mix 500 grams of polyphosphoric acid and 250 grams of phosphoric acid, and add 75 grams of raw material phenylhydrazine pyruvic acid phenylhydrazone in batches while heating to 50°C under stirring, and control the temperature in the range of 70~90°C for 0.5 hours After adding the raw materials, continue the temperature control reaction for 20 minutes, monitor the completion of the reaction, pour it into 1500 grams of ice-water mixture, cool, filt...
example 2
[0040] Example 2. Synthesis of 5-fluoroindole-2-carboxylic acid
[0041] Step 1: Using ethanol as the solvent, heat 162.5 g of 4-fluorophenylhydrazine hydrochloride and 127 g of ethyl pyruvate in a mixed solvent of 1205 ml of ethanol and 241 ml of water, and reflux at 80°C for 4 hours After monitoring the completion of the reaction and removing the solvent, it was recrystallized with a 70% ethanol aqueous solution, filtered, and dried to obtain 186.1 g of pale yellow crystals of 4-fluorophenylhydrazine pyruvate phenylhydrazone with a yield of 83%.
[0042] Step 2: Mix 500 g of polyphosphoric acid and 250 g of phosphoric acid, and under the condition of heating to 75°C under stirring, add 65 g of 4-fluorophenylhydrazine pyruvic acid phenylhydrazone in batches, and control the temperature in the range of 75~85°C After adding the raw materials in 1 hour, continue the temperature control reaction for 20 minutes, monitor the completion of the reaction, pour it into 1500 grams of ice-wat...
example 3、5
[0045] Example 3. Synthesis of 5-chloroindole-2-carboxylic acid
[0046] Using ethanol as the solvent, heat 179.05 g 4-chlorophenylhydrazine hydrochloride and 127 g ethyl pyruvate in a mixed solvent of 1225 ml methanol and 265 ml water, reflux at a temperature of about 80°C, and monitor the reaction for 3 hours After the solvent was removed, it was recrystallized with 70% ethanol aqueous solution, filtered, and dried to obtain 219 grams of 4-chlorophenylhydrazine pyruvic acid phenylhydrazone with a yield of 91%.
[0047] Step 2: Mix 500 grams of polyphosphoric acid and 250 grams of phosphoric acid, heat to 70°C under stirring, add 70 grams of 4-chlorophenylhydrazine pyruvate phenylhydrazone in batches, and control the temperature in the range of 70~90°C for 1 hour After adding the raw materials, continue the temperature control reaction for 40 minutes, monitor the completion of the reaction, pour it into 1500 grams of ice-water mixture, cool, filter and dry to obtain a pale yellow ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com