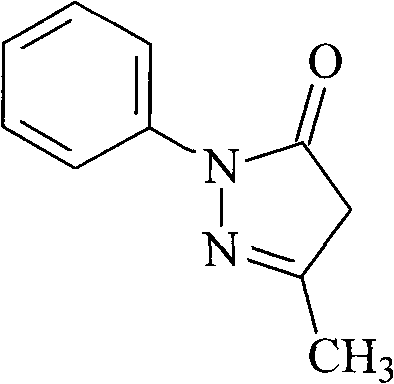
Edaravone compound synthesized by new method
A compound and route technology, applied in the field of medicine, can solve the problems of slow reaction, low yield, and difficulty in obtaining butanone amide, and achieve the effects of reducing production cost and improving yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
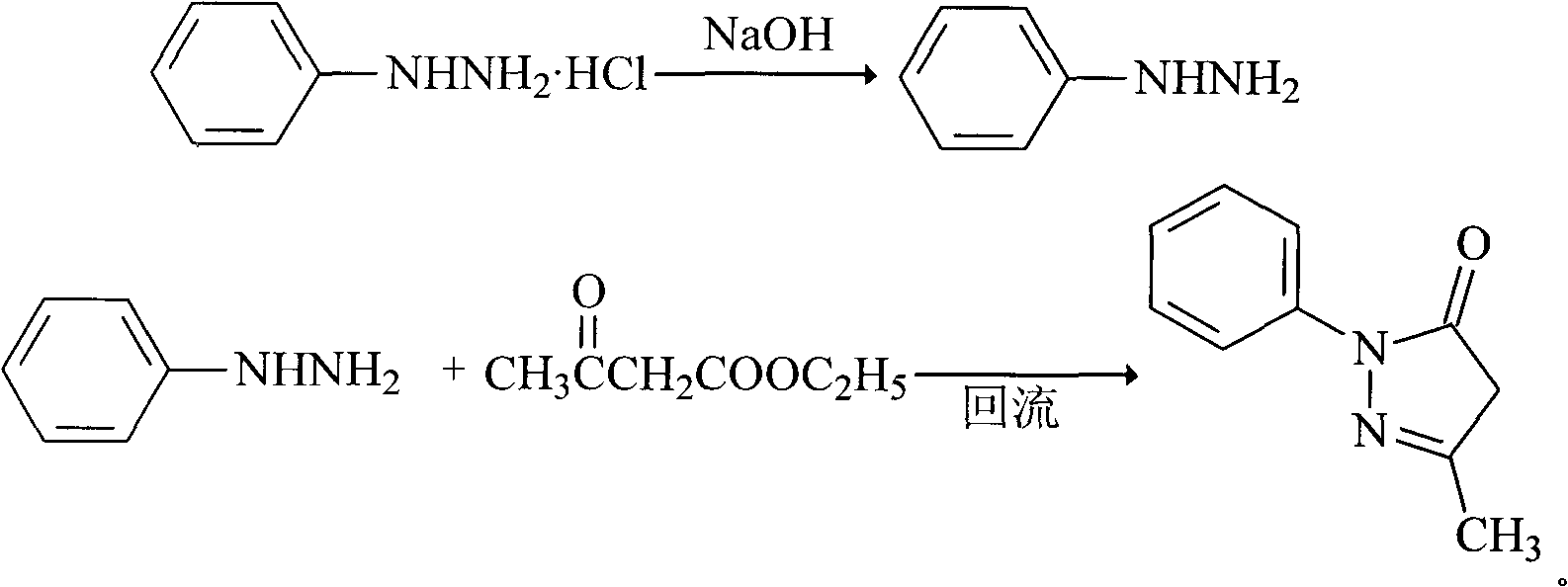
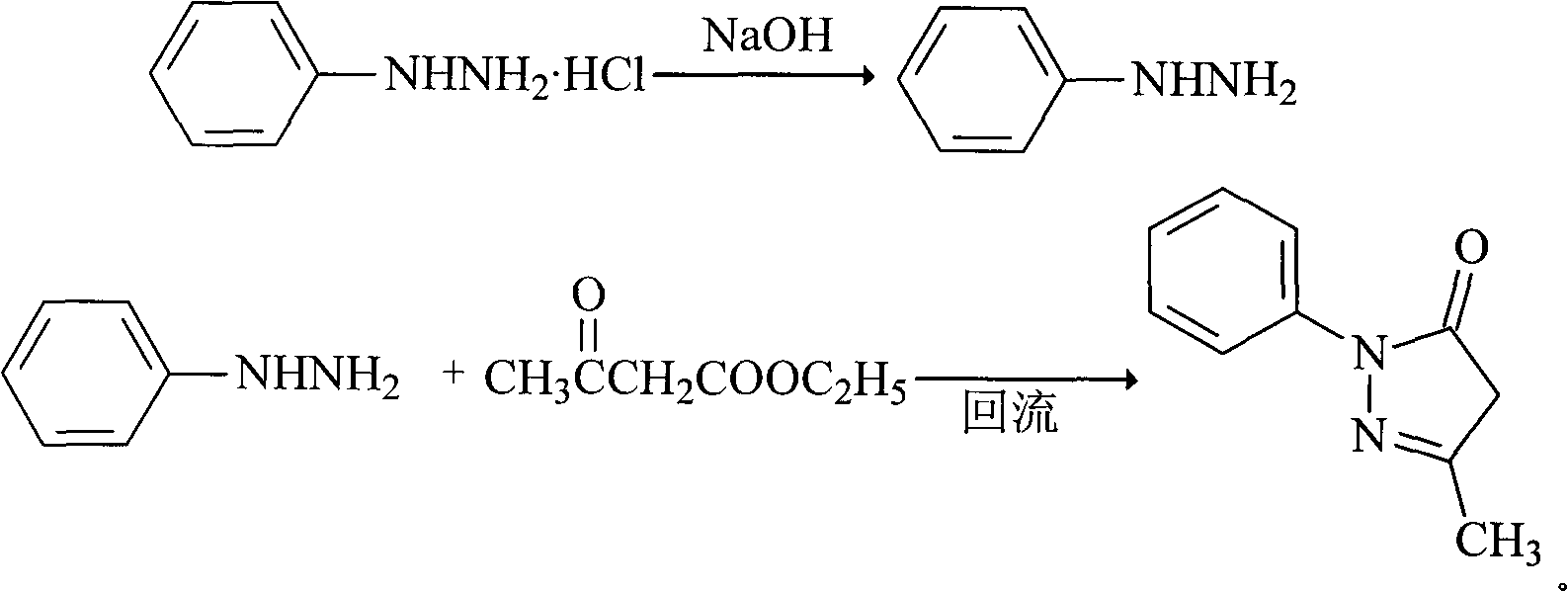
[0023] The synthesis of embodiment 1 Edaravone
[0024] (1) Weigh 13.5g of phenylhydrazine hydrochloride (94mmol), add it to 100ml of water, stir for 0.5 hour, add equimolar sodium hydroxide 3.76g, stir for 0.5 hour;
[0025] (2) Add 11.7g of ethyl acetoacetate (90mmol) dropwise to the above reaction solution, the reaction exotherms, heat up to reflux for 2.5 hours, stop heating, stir and cool to room temperature, filter, and dry to obtain light yellow granular crude product 15.5 g;
[0026] (3) Add 30ml of isopropanol-water solution with a volume ratio of 2:1 to the above crude product, add 2g of activated carbon, reflux for 1 hour, heat filter, cool to room temperature and precipitate a white solid to obtain 14.8g of white crystalline powder, yield It is 90%, mp129°C, and the purity is 99.9%.
Embodiment 2
[0027] The synthesis of embodiment 2 Edaravone
[0028] (1) Weigh 15g of phenylhydrazine hydrochloride (104mmol), add it to 120ml of water, stir for 0.5 hour, add equimolar sodium hydroxide 4.16g, stir for 0.5 hour;
[0029] (2) Add 13g of ethyl acetoacetate (100mmol) dropwise to the above reaction solution, exothermic reaction, heat up to reflux reaction for 2.5 hours, stop heating, stir and cool to room temperature, filter, and obtain 16.7g of light yellow granular crude product after drying ;
[0030] (3) Add 40ml of the above crude product into isopropanol-water solution with a volume ratio of 2:1, add 2.5g of activated carbon, reflux for 1 hour, heat filter, cool to room temperature and precipitate a white solid to obtain 16.1g of white crystalline powder, which is collected The yield is 88.9%, the mp is 128°C, and the purity is 99.9%.
Embodiment 3
[0031] The synthesis of embodiment 3 Edaravone
[0032] (1) Weigh 22g of phenylhydrazine hydrochloride (152mmol), add it to 200ml of water, stir for 0.5 hour, add equimolar sodium hydroxide 6.08g, stir for 0.5 hour;
[0033] (2) Add 19g of ethyl acetoacetate (146mmol) dropwise to the above reaction solution, the reaction exotherms, heat up to reflux for 3 hours, stop heating, stir and cool to room temperature, filter, and dry to obtain 24.8g of light yellow granular crude product ;
[0034] (3) Add 50ml of the above crude product into isopropanol-water solution with a volume ratio of 2:1, add 3g of activated carbon, reflux for 1 hour, heat filter, cool to room temperature and precipitate a white solid to obtain 23.2g of white crystalline powder, yield It is 87.8%, mp128°C, and the purity is 99.9%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com