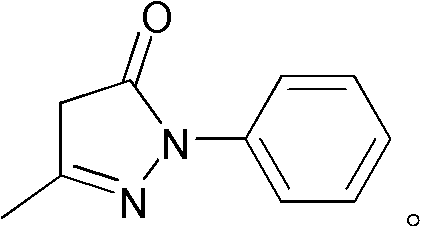
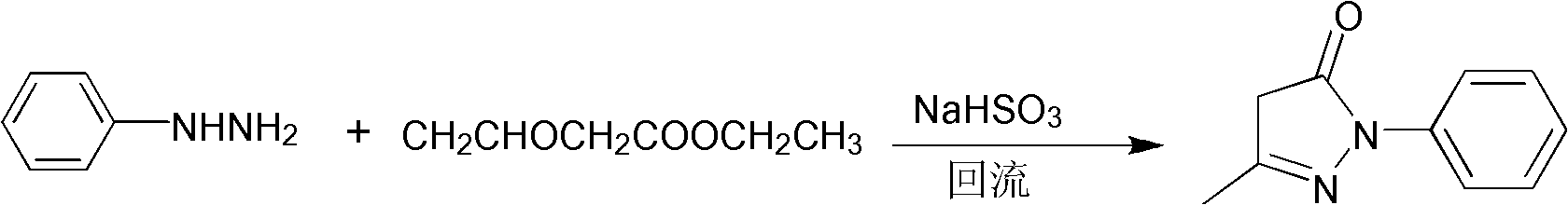A kind of optimized Edaravone synthetic method
A synthesis method and solution technology, applied in the field of medicinal chemistry, can solve the problems of low reaction yield and achieve high yield, low cost, and improved purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
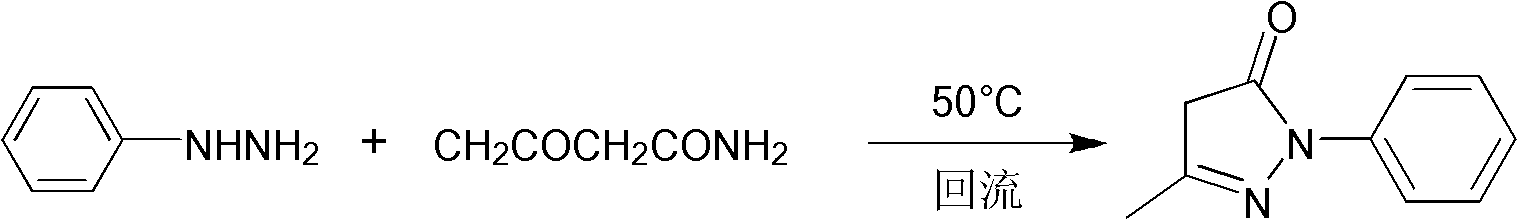
[0026] Embodiment 1: Edaravone is prepared from phenylhydrazine.
[0027] a. Weigh 5.1 g of phenylhydrazine (47 mmol), add it into a round bottom flask filled with 45 mL of water under stirring, take an appropriate amount of concentrated hydrochloric acid, measure and adjust the pH value of the solution to 6.0 with a pH meter.
[0028] b. Slowly add 5.85 g (45 mmol) of ethyl acetoacetate dropwise to the above solution, the reaction is exothermic, and after cooling down to room temperature, add 1.5 g of sodium dithionite (Na 2 S 2 o 4 ), heated to 105° C. and refluxed for 3 hours, then stopped heating, then stirred, cooled, suction filtered, and dried to obtain the crude product of pale yellow granular Edaravone.
[0029] c. Recrystallize with absolute ethanol, filter with suction, and dry to obtain white crystalline powder, which is refined Edaravone, with a yield of 85% and a purity of 99.2%.
Embodiment 2
[0030] Embodiment 2: Edaravone is prepared from phenylhydrazine hydrochloride.
[0031] a. Weigh 6.8 g of phenylhydrazine hydrochloride (47 mmol), add it into a round-bottom flask filled with 45 mL of water under stirring, and adjust the pH value of the solution to 6.0 with ammonia water.
[0032] b. Slowly add 5.85g (45mmol) of ethyl acetoacetate dropwise to the above solution. 2 S 2 o 4 ), heated to 105° C. and refluxed for 3 hours, then stopped heating, then stirred, cooled, suction filtered, and dried to obtain the crude product of pale yellow granular Edaravone.
[0033] c. Recrystallize with absolute ethanol, filter with suction, and dry to obtain white crystalline powder, which is refined Edaravone, with a yield of 84% and a purity of 99.2%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com