Green synthetic method of indole carbazole compounds for organic electroluminescent materials
An electroluminescent material, indolocarbazole technology, applied in the direction of organic chemistry, etc., can solve the problems of high cost, difficult to obtain diindolylmethane, etc., and achieve the effects of less impurities, easily available price, and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
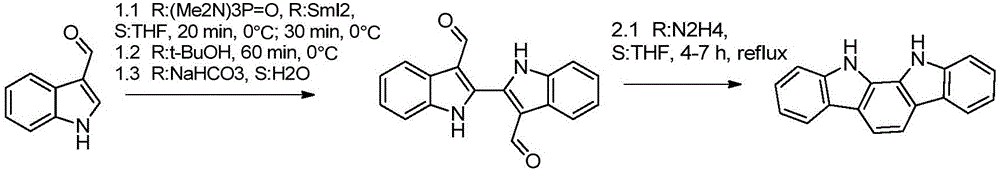
[0022] Add 500ml of decahydronaphthalene, 22.4g of 1,2-cyclohexanedione, and 57.6g of phenylhydrazine hydrochloride into a 1L three-neck flask, heat to control the temperature (190°C±2), stop the reaction after 30 minutes, and solvent recovery cycle used in the reaction process. After cooling down to room temperature, a large amount of products were precipitated, filtered to obtain 47.2 g of (1) 11,12-dihydroindolo[2,3-a]carbazole in the form of khaki powder, melting point: 367-375°C (yield: 92.1 %, HPLC=99.7%).
[0023] 1 HNMR (400MHz, DMSO-d6) δ11.36 (2H, s), 8.16 (2H, d), 7.92 (2H, s), 7.73 (2H, d), 7.51 (2H, m), 7.26 (2H, m )
Embodiment 2
[0025] Add 500ml of diphenyl ether, 622.4g of 1,3-cyclohexanedi, 57.6g of phenylhydrazine hydrochloride into a 1L three-necked flask, heat to control the temperature (190°C±2), stop the reaction after 30 minutes, and recover the solvent for recycling in the reaction process. After cooling down to room temperature, a large amount of products precipitated, filtered to obtain (2) 5,12-dihydroindolo[3,2-a]carbazole 46.3g (MS: 128,256) off-white powder. Melting point: 298-305°C (Yield: 90.3%, HPLC=99.5%). 1 H NMR (400 MHz, DMSO-d6) δ 11.06 (2H, s), 8.26 (3H, d), 7.81 (2H, d), 7.62 (2H, m), 7.23 (3H, m).
Embodiment 3
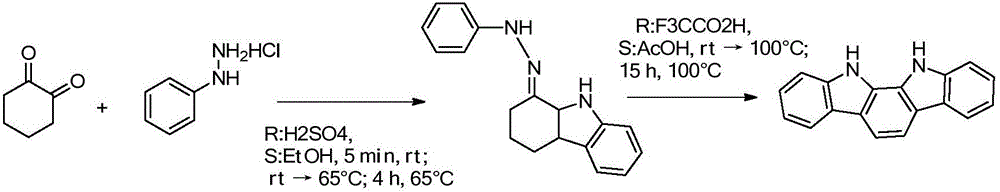
[0027] Add 250ml of decahydronaphthalene, 250ml of diphenyl ether, 22.4g of 1,4-cyclohexanedione, and 57.6g of phenylhydrazine hydrochloride into a 1L three-necked flask, heat to control the temperature (190°C±2), and stop after 30 minutes. Reaction, solvent recovery and recycling for the reaction process. When the temperature was lowered to room temperature, a large amount of products were precipitated. After filtration, 47.1 g of (3) 5,11-dihydroindolo[3,2-b]carbazole was obtained as off-white powder (91.9% yield, HPLC=99.7%).
[0028] 1 HNMR (400 MHz, DMSO-d6) δ 11.03 (2H, s), 8.21 (2H, d), 7.46 (2H, d), 7.36 (2H, t), 7.12 (2H, t).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com