Amphiphilic indole squarylium cyanine dye and application thereof in long-acting marking of lysosome
An indole squaraine and amphiphilic technology, applied in the field of synthesis and its application in specific lysosome labeling, can solve the problems of inability to label subcellular organelles, increase biological toxicity, and short labeling time, and achieve product Good stability, low biological toxicity, strong electrostatic effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
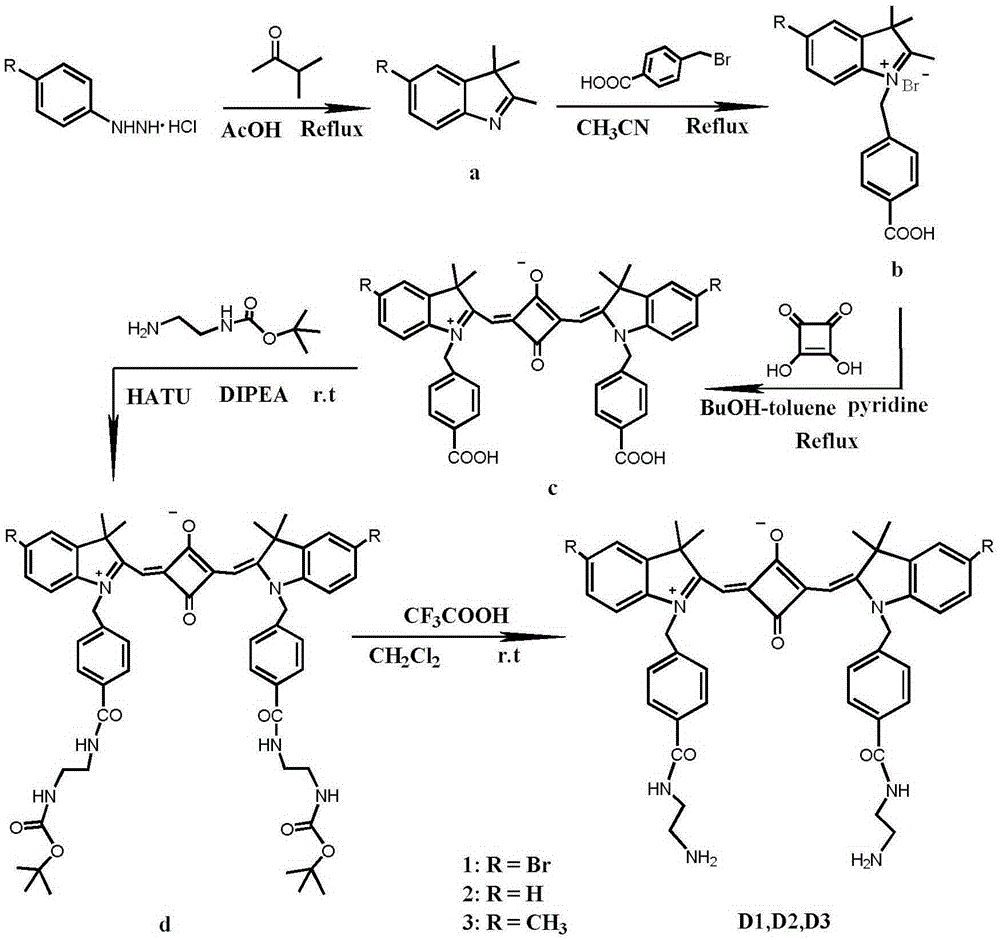
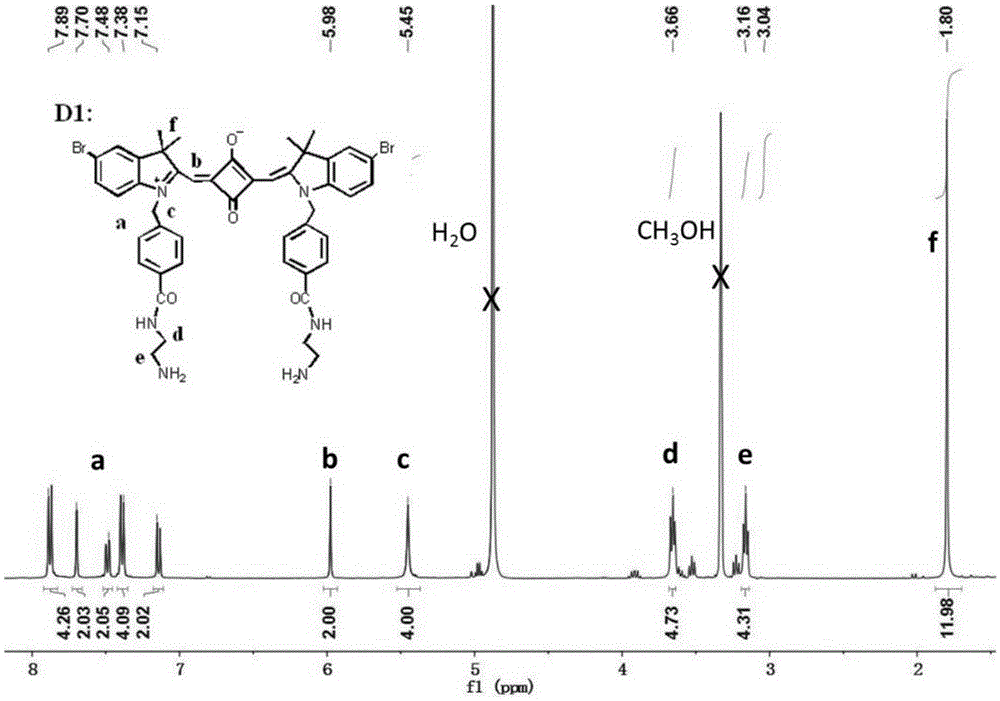
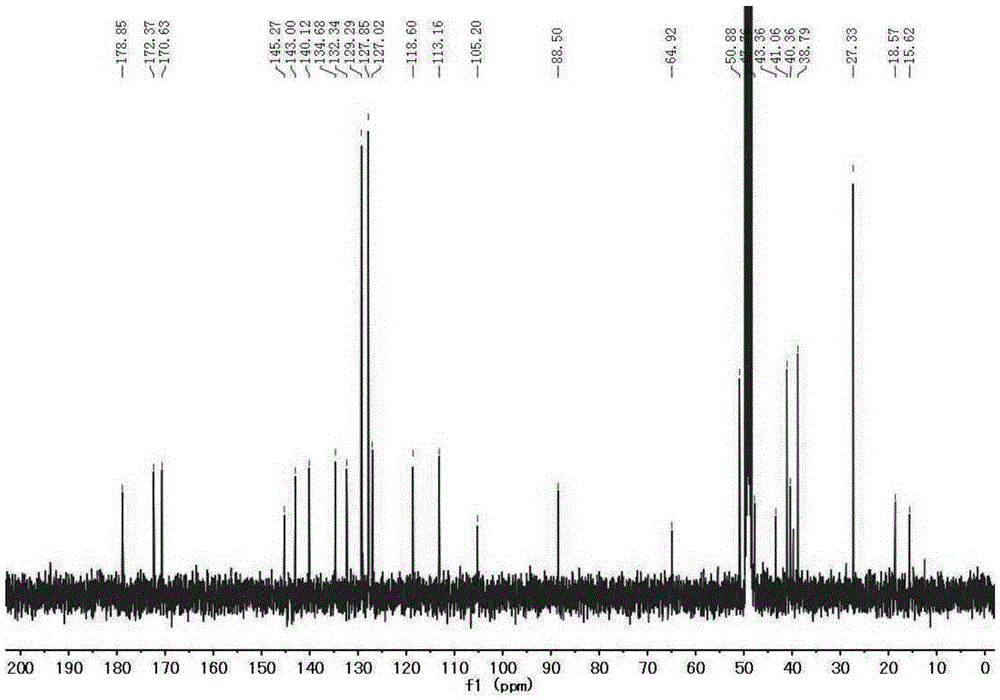
[0035] 1. Add 2.23g (10mmol) of 4-bromophenylhydrazine hydrochloride and 0.86g (10mmol) of 3-methyl-2-butanone into 10mL of glacial acetic acid, reflux for 12 hours, and evaporate to remove the solvent. Dissolve with dichloromethane and wash with saturated NaHCO 3 The aqueous solution was washed repeatedly until the aqueous phase was colorless and the pH was neutral, and the organic phase was removed by rotary evaporation to obtain 1.88 g (7.9 mmol) of 5-bromo-2,3,3-trimethyl-3H-ylindole with a yield of 79%. ;
[0036] 1 HNMR (400MHz, CDCl 3 )δ7.40(s,3H),2.27(s,3H),1.30(s,6H).
[0037] 2. Add 1.19g (5mmol) of 5-bromo-2,3,3-trimethyl-3H-ylindole and 1.08g (5mmol) of p-benyl bromobenzoic acid to 15mL of acetonitrile, reflux for 20 hours, and remove by rotary evaporation After the solvent was precipitated with ether and washed several times, 1.72 g (3.8 mmol) of the intermediate indoline derivative was obtained after drying, with a yield of 76%;
[0038] 1 HNMR(400MHz,MeOD)...
Embodiment 2
[0053] 1. Add 1g (4.5mmol) of phenylhydrazine hydrochloride and 0.54 (6.3mmol) of 3-methyl-2-butanone into 10mL of glacial acetic acid, reflux for 12 hours, remove the solvent by suspension evaporation, and use dichloro Dissolved in methane and washed with saturated NaHCO 3 The aqueous solution was washed repeatedly until the aqueous phase was colorless and the pH was neutral, and the organic phase was removed by rotary evaporation to obtain 0.77 g of 2,3,3-trimethyl-3H-ylindole with a yield of 71%;
[0054] 2. Add 0.72g (3mmol) of 2,3,3-trimethyl-3H-ylindole and 0.96g (4.5mmol) of p-benyl bromobenzoic acid to 15mL of acetonitrile, reflux for 20 hours, remove the solvent by rotary evaporation and use Diethyl ether precipitated and washed several times, and after drying, 0.96 g of the intermediate indoline derivative was obtained, with a yield of 72%;
[0055] 3. Add 836mg (2mmol) of indoline derivatives and 114mg (1mmol) of 3,4-dihydroxy-3-cyclobutene-1,2-dione (squaric acid)...
Embodiment 3
[0062] 1. Add 500mg (3.2mmol) of 4-methylphenylhydrazine hydrochloride and 430mg (5mmol) of 3-methyl-2-butanone into 10mL of glacial acetic acid, reflux for 12 hours, and remove the solvent by suspension evaporation. Dissolve with dichloromethane and wash with saturated NaHCO 3 The aqueous solution was washed repeatedly until the aqueous phase was colorless and the pH was neutral, and the organic phase was removed by rotary evaporation to obtain 450 mg (2.6 mmol) of 5-methyl-2,3,3-trimethyl-3H-ylindole, with a yield of 81%. ;
[0063] 2. Add 340mg (1.96mmol) of 5-methyl-2,3,3-trimethyl-3H-ylindole and 422mg (1.96mmol) of p-benyl bromobenzoic acid to 10mL of acetonitrile, reflux for 20 hours, and rotate to evaporate After removing the solvent, it was precipitated with ether and washed several times, and after drying, 608 mg (1.57 mmol) of the intermediate indoline derivative was obtained, with a yield of 80%;
[0064] 3. Add 155 mg (0.4 mmol) of indoline derivatives and 25 mg...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com