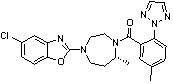
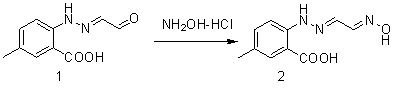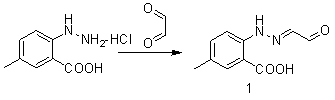Synthetic method for anti-sleeplessness medicine MK-4305 intermediate
A technology of MK-4305 and a synthetic method, applied in the field of organic and pharmaceutical synthesis, can solve the problems of high preparation cost, harsh separation conditions, complicated operation and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] (1) Synthesis of Compound 1
[0024]
[0025] Add 4-methyl-2-carboxyphenylhydrazine hydrochloride (2.0g, 0.0115mol) dissolved in acetic acid (70ml) into a 500mL two-necked flask, dilute glyoxal (2.1g, 0.0362mol) with 50mL of water, Slowly add this solution dropwise into the two-necked flask under ice bath; after the dropwise addition, continue to stir for half an hour; filter, 50 o C was dried in vacuo to obtain 2.35 g of compound 1. The yield was 99.0%. It can be directly used in the next reaction without purification.
[0026] (2) Synthesis of Compound 2
[0027]
[0028] Add compound 1 (2.35 g, 0.0114mol) and 30mL ethanol into a 100mL two-necked bottle, stir until dissolved, add hydroxylamine hydrochloride (3.26g, 0.0472mol) under ice cooling; then add 2M sodium hydroxide aqueous solution (2.5ml) dropwise, Continue to stir for 1h after dripping, filter, 50 o C was dried in vacuo to obtain 2.4 g of compound 2 with a yield of 95.2%. It can be directly used ...
Embodiment 2
[0036] (1) Synthesis of Compound 1
[0037]
[0038] Add 4-methyl-2-carboxyphenylhydrazine hydrochloride (2.0g, 0.0115mol) dissolved in 6M hydrochloric acid (70ml) into a 500mL two-necked bottle, take glyoxal (2.1g, 0.0362mol) and add 50mL of water to dilute , slowly drop this solution into the two-necked bottle at room temperature; after the dropwise addition, continue to stir for half an hour; filter, 50 o C was dried in vacuo to obtain 2.25 g of compound 1. The yield was 94.5%. It can be directly used in the next reaction without purification.
[0039] (2) Synthesis of Compound 2
[0040]
[0041] Add compound 1 (2.25 g, 0.0109 mol) and 30 mL of methanol into a 100 mL two-necked flask, stir until dissolved, add hydroxylamine hydrochloride (3.26 g, 0.0472 mol) under ice cooling; then add 2M potassium hydroxide aqueous solution (2.5 mL) dropwise, Continue to stir for 1h after dripping, filter, 50 o C was dried in vacuo to obtain 2.2 g of compound 2 with a yield of ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com