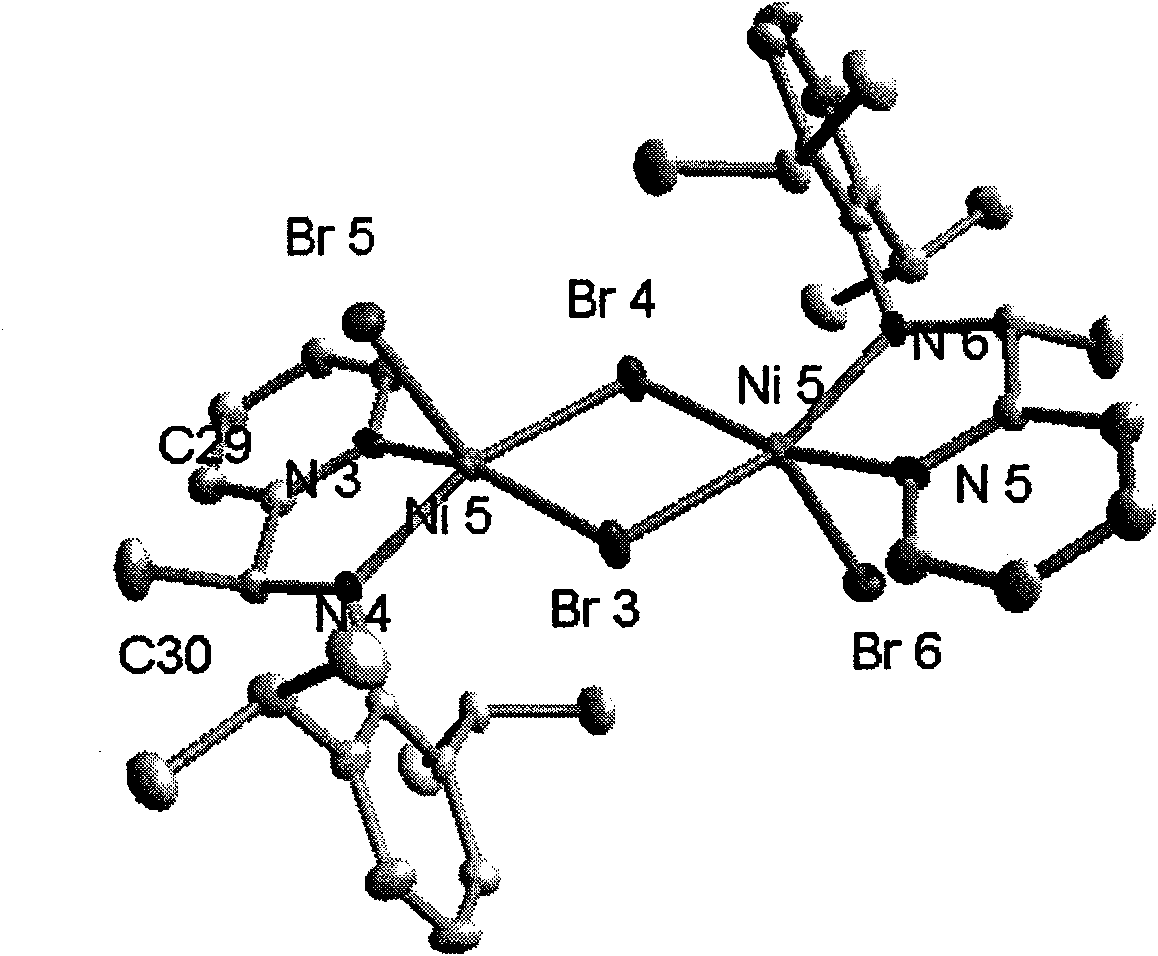
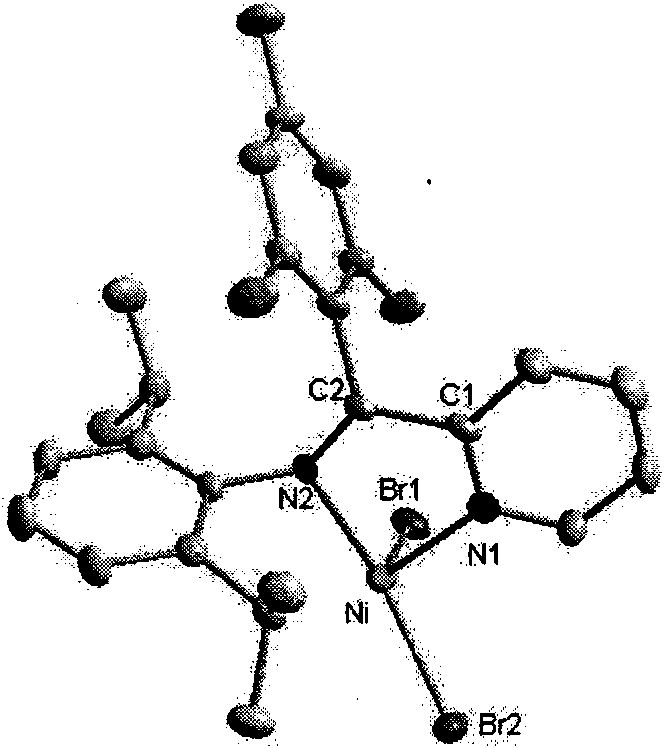
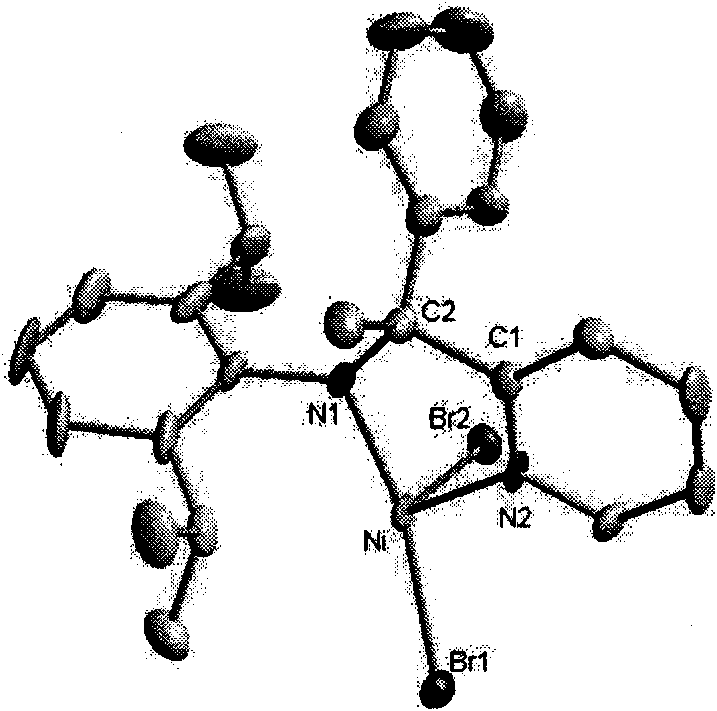
2-ammonia methyl-pyridine nickel composition, preparation method and application thereof
A technology of aminomethylpyridine nickel and complexes, applied in nickel organic compounds, organic chemistry, etc., to achieve the effect of inhibiting chain transfer and chain termination
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0044] Synthesis of 2-pyridine imine compound A1:
[0045] Dissolve 10mL (0.048mol) of 2,6-diisopropylaniline in 60mL of ethanol, N 2 Protect. Then slowly add 4.8 mL (0.048 mol) of 2-formylpyridine and 5 drops of formic acid, and stir and reflux at 80°C for 3 hours. After cooling to room temperature, ethanol was removed under reduced pressure, and recrystallized with n-hexane. 11 g of yellow crystals were obtained, yield: 85.8%. The results of hydrogen spectroscopy are as follows: 1 H NMR (300MHz, CDCl 3 ): δ1.25-1.27 (12H, d, 4CH3), 3.34-3.43 (2H, m, 2CH), 7.50 (1H, S, CH), 7.05-7.14 (3H, m, benzyl), 7.18-7.22 (1H , t, Py-H), 7.30-7.32 (1H, d, Py-H), 7.62-7.68 (1H, t, Py-H), 8.61-8.63 (1H, d, Py-H). The product was determined to be 2-pyridine imine compound A1.
Embodiment 2
[0047] Synthesis of 2-pyridine imine compound A2
[0048] Under nitrogen protection, add 4.2503g (24mmol) 2,6-diisopropylaniline, and 20mL toluene to a branch bottle equipped with a condenser tube, inject 8mL of 3M trimethylaluminum toluene solution with a syringe, and heat to 120°C and stirred for 2 hours. After cooling, 3.6688 g (24 mmol) of phenyl(2-pyridyl)methanone was dissolved in 8 mL of toluene, and then the above solution was added. Heating was continued at 120°C for 6 hours, then the temperature was adjusted to 70°C and stirred overnight. Distilled water was added under stirring to terminate the reaction, extracted three times with ethyl acetate, and the organic phases were combined. The organic phase was washed once with distilled water, anhydrous Na 2 SO 4 Dry, filter, and spin dry in vacuo. Recrystallized from ethanol to obtain 6.8413 g of the product with a yield of 81%. The results of hydrogen spectroscopy are as follows: 1 HNMR (300MHz, CDCl 3 ): δ0.91-...
Embodiment 3
[0050] Synthesis of 2-pyridine imine compound A3
[0051] Dissolve 10mL (0.05mol) of 2,6-diisopropylaniline in 60mL of ethanol, N 2 Protect. Then 8.7 g (0.05 mol) of 2-formyl-6-phenylpyridine and 5 drops of formic acid were added slowly, and stirred and refluxed at 80° C. for 3 hours. After cooling to room temperature, ethanol was removed under reduced pressure, and recrystallized with n-hexane. 10 g of yellow crystals were obtained, yield: 62.5%. Mass spectrometry determined that the product was 2-pyridine imine compound A3.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Catalytic efficiency | aaaaa | aaaaa |
| Catalytic efficiency | aaaaa | aaaaa |
| Catalytic efficiency | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com