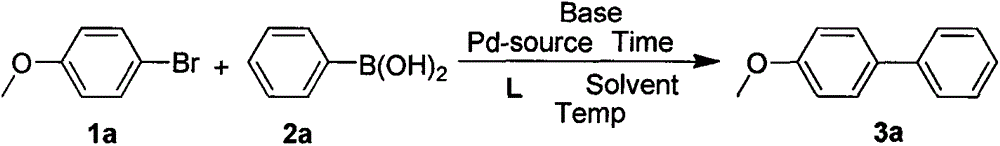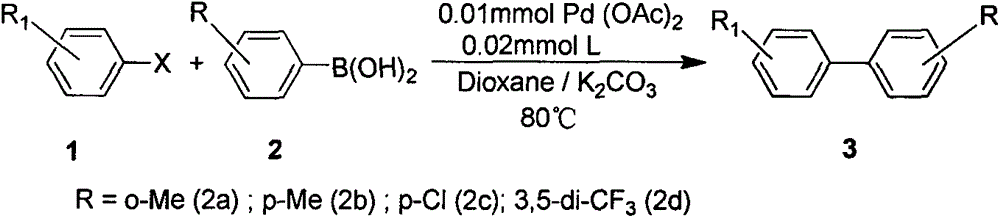Synthetic method and application of novel multi-aryl bridged long-chain diphosphine ligand
A long-chain diphosphine ligand technology, which is applied in the field of preparation of catalyst ligands, can solve the problems of phosphine ligand design that have not been reported before, and achieve the effects of small amount of catalyst, convenient post-treatment, and high reactivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
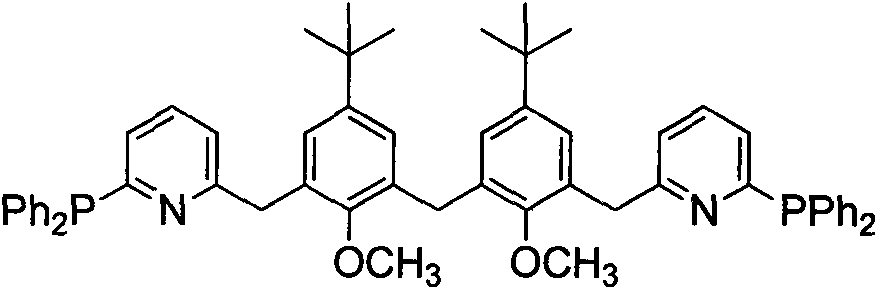
[0030] A novel polyaryl bridged long-chain diphosphine ligand bis[3-(6-(2-diphenylphosphino)pyridinyl)-5-tert-butyl-2-methyl The synthetic method of oxyphenyl] methane is as follows:
[0031] (1) Preparation of bis(3-bromomethylene-5-tert-butyl-2-methoxyphenyl)methane
[0032] 1) Add 5.00 g (13.43 mmol) of bis(5-tert-butyl-3-hydroxymethyl-2-hydroxyphenyl)methane and acetone solvent to a 150 ml three-necked flask equipped with mechanical stirring, reflux condenser and thermometer 40ml, fully dissolved under stirring, add K 2 CO 3 12.00g (0.087mol), heated to reflux, stirred and reacted for 1h under reflux conditions, added CH 3 5.00ml of acetone solution of 13.30ml (0.053mol), stirred and reacted under reflux, monitored by TLC, and the reaction was completed in about 8h. Stop heating, filter while hot, and concentrate the filtrate to remove the solvent, then use CH 2 Cl 2 Extraction, washing with saturated NaCl aqueous solution, liquid separation, organic phase with anhyd...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com