Catalyst system and catalyzing method of propylene hydrogenation and formylation
A propylene hydroformylation catalysis and system technology, applied in chemical instruments and methods, organic compound/hydride/coordination complex catalysts, physical/chemical process catalysts, etc., can solve the problem of low butyraldehyde normal/iso ratio , Expensive epoxy compounds, accelerated butyraldehyde polymerization, etc., to achieve high activity and selectivity, prolong service life, and reduce dosage
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
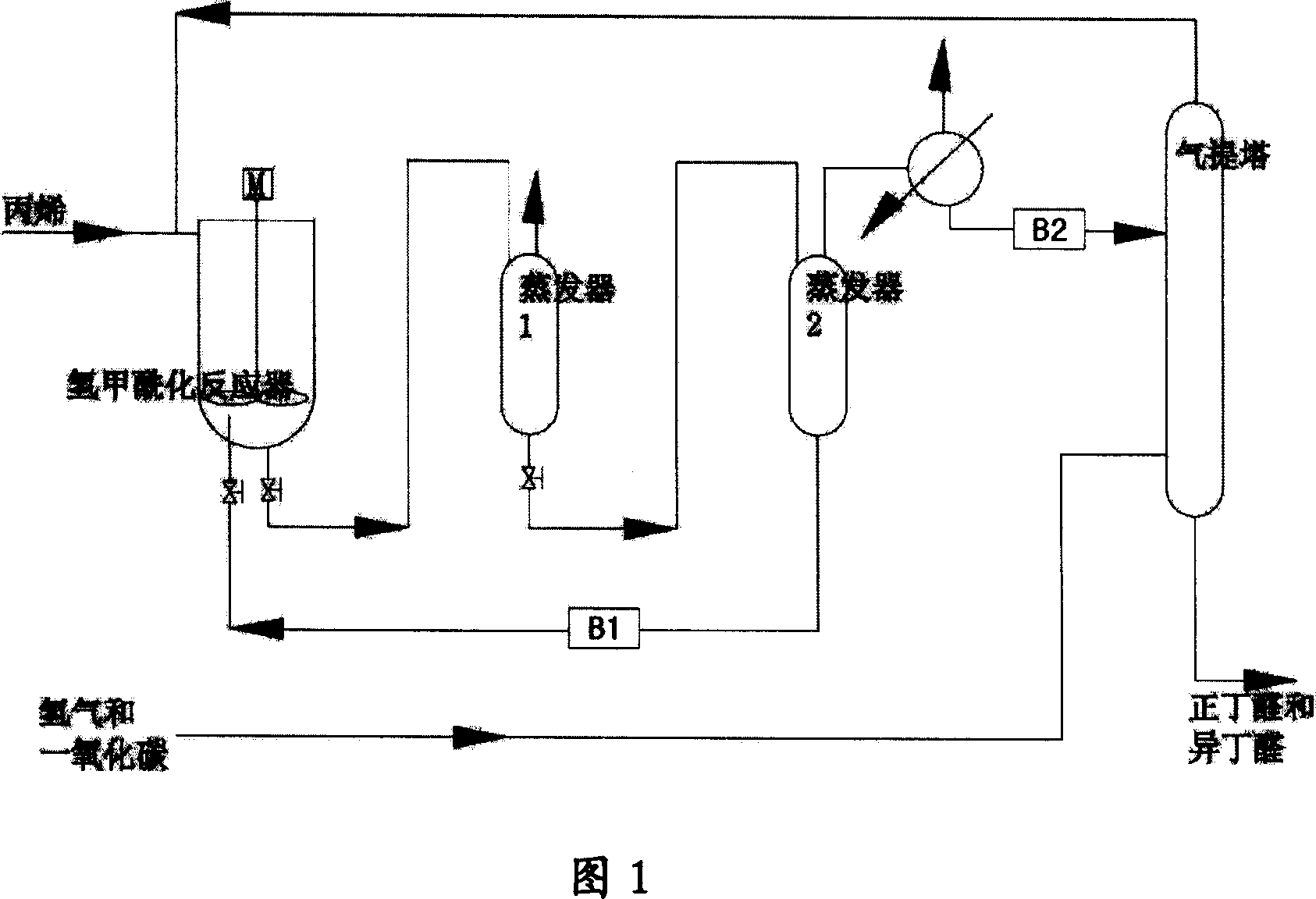
Image
Examples
Embodiment 1
[0040]
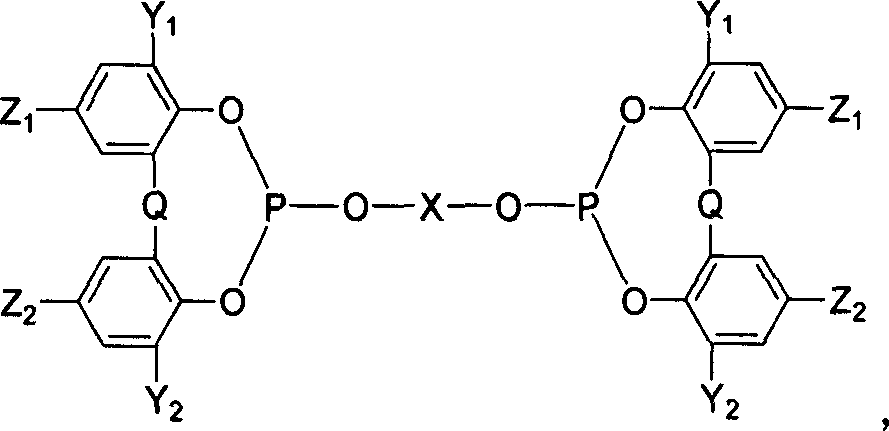
[0041] Synthesis of bidentate phosphite L1: addition of 2,2'-biphenol to excess PCl 3 In, after heating to reflux (reflux) for 5 hours, the excess PCl was distilled off under reduced pressure 3 , to obtain white solid compound A (yield 71%). A solution of 2,2'-biphenol (0.37 g, 2 mmol) and triethylamine (2.8 mL, 20 mmol) in tetrahydrofuran (THF) was added dropwise to a solution of A (1.25 g, 5 mmol) in THF at ice bath temperature After the dropwise addition, it was gradually raised to room temperature (r.t,) and stirred for 24 hours before processing, and recrystallized after silica gel column purification to obtain the product L1, white crystals, the yield was 90% (1.1g), 1 H NMR and 31 The structure was identified by P NMR, and the melting point was 124°C.
Embodiment 2
[0043]
[0044] Synthesis of bidentate phosphite L2:
[0045] At -40°C, a solution of binaphthyldiol (1.43g, 5mmol) and triethylamine (5.6mL, 40mmol) in tetrahydrofuran was added dropwise to PCl 3 (0.43mL, 5mmol) in tetrahydrofuran solution, gradually warmed up to room temperature after the dropwise addition, cooled to -40°C after stirring for 2 hours, then added dropwise Compound B (0.82g, 2mmol) and triethylamine (2.8mL, 20mmol ) tetrahydrofuran solution, after the dropwise addition, the temperature was gradually raised to room temperature, and after stirring for 2 hours, it was processed and purified through a silica gel column to obtain the product L2, a white solid, with a yield of 67% (1.39g), 1 H NMR and 31 The structure was identified by P NMR with a melting point of 249-251°C.
Embodiment 3
[0047]
[0048] Synthesis of bidentate phosphite L3: At room temperature, a toluene solution of compound B (1.0 g, 2.5 mmol) and triethylamine (2.8 mL, 20 mmol) was added dropwise to PCl 3 (0.22mL, 2.5mmol) in toluene solution, heat up and reflux for 2 hours, then cool to room temperature, then add dropwise the toluene solution of 2,2'-biphenol (0.18g, 1mmol) and triethylamine (1.4mL, 10mmol) , after the dropwise addition, the temperature was raised to reflux for 2 hours, cooled and processed, and purified through a silica gel column to obtain the product L3, a white solid, with a yield of 30% (0.31g), 1 H NMR and 31 The structure was identified by P NMR with a melting point of 145-147°C.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com