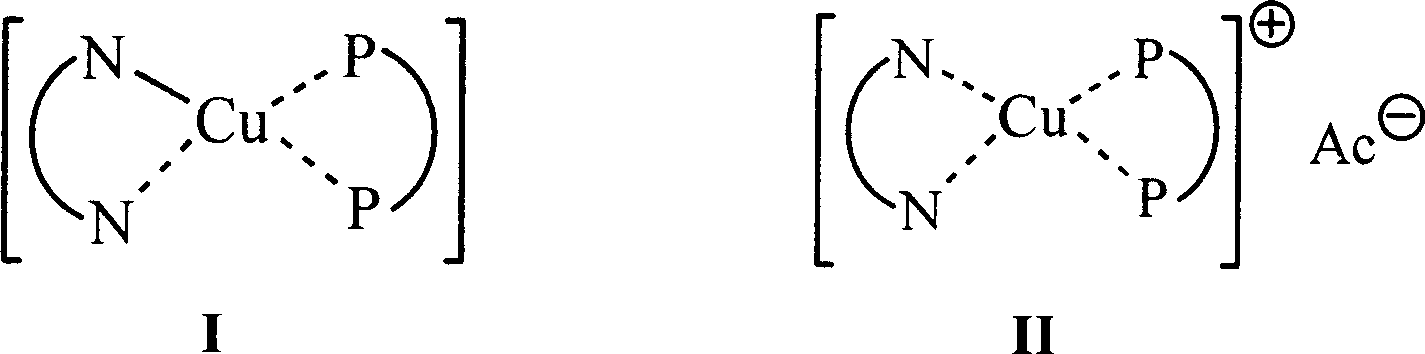Copper (1) compound with imidazole derivate as compounding body
A technology of imidazole derivatives and complexes, which is applied in the field of copper complexes, can solve problems such as time delay, and achieve the effect of easy synthesis and purification
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
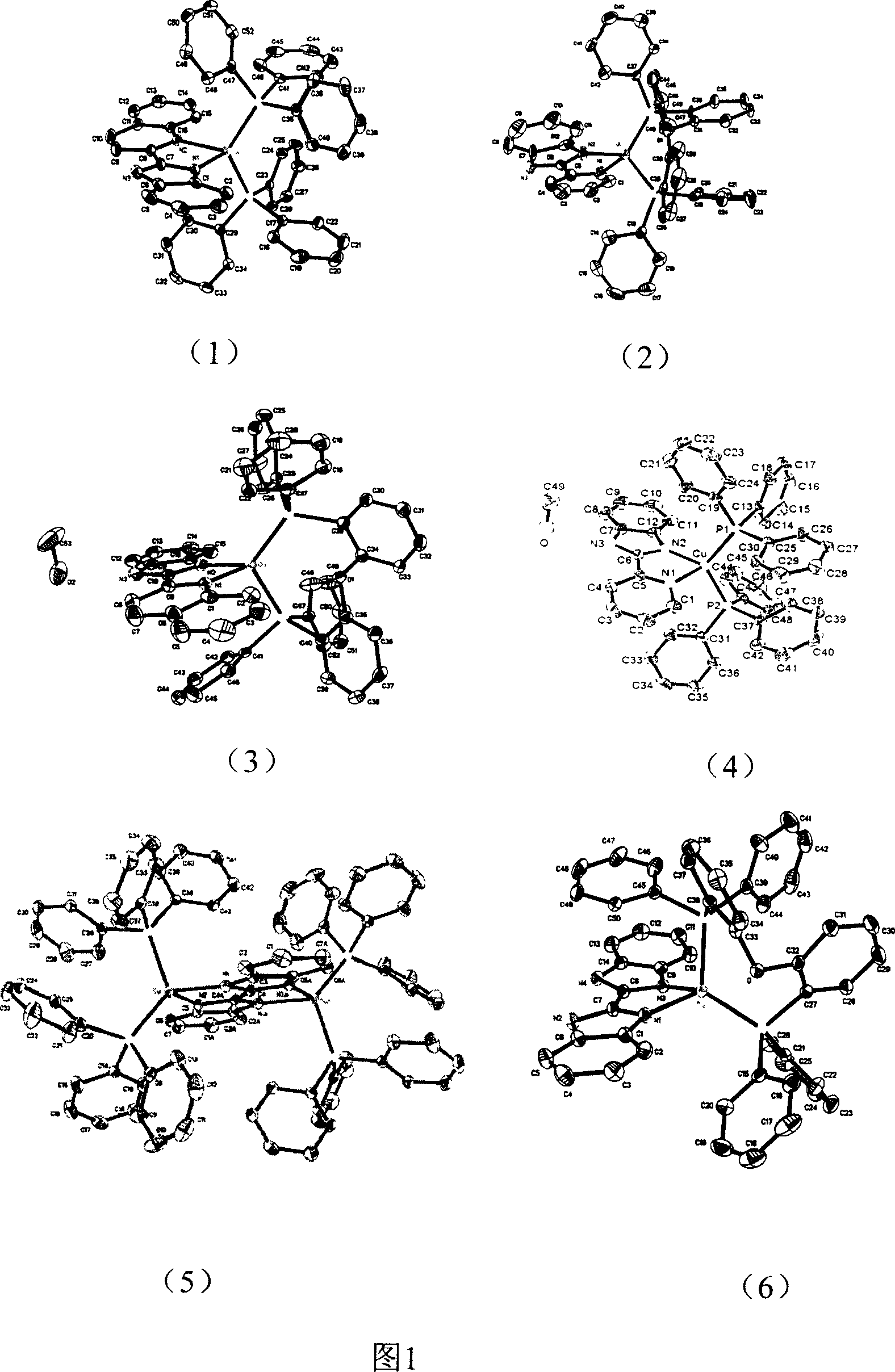
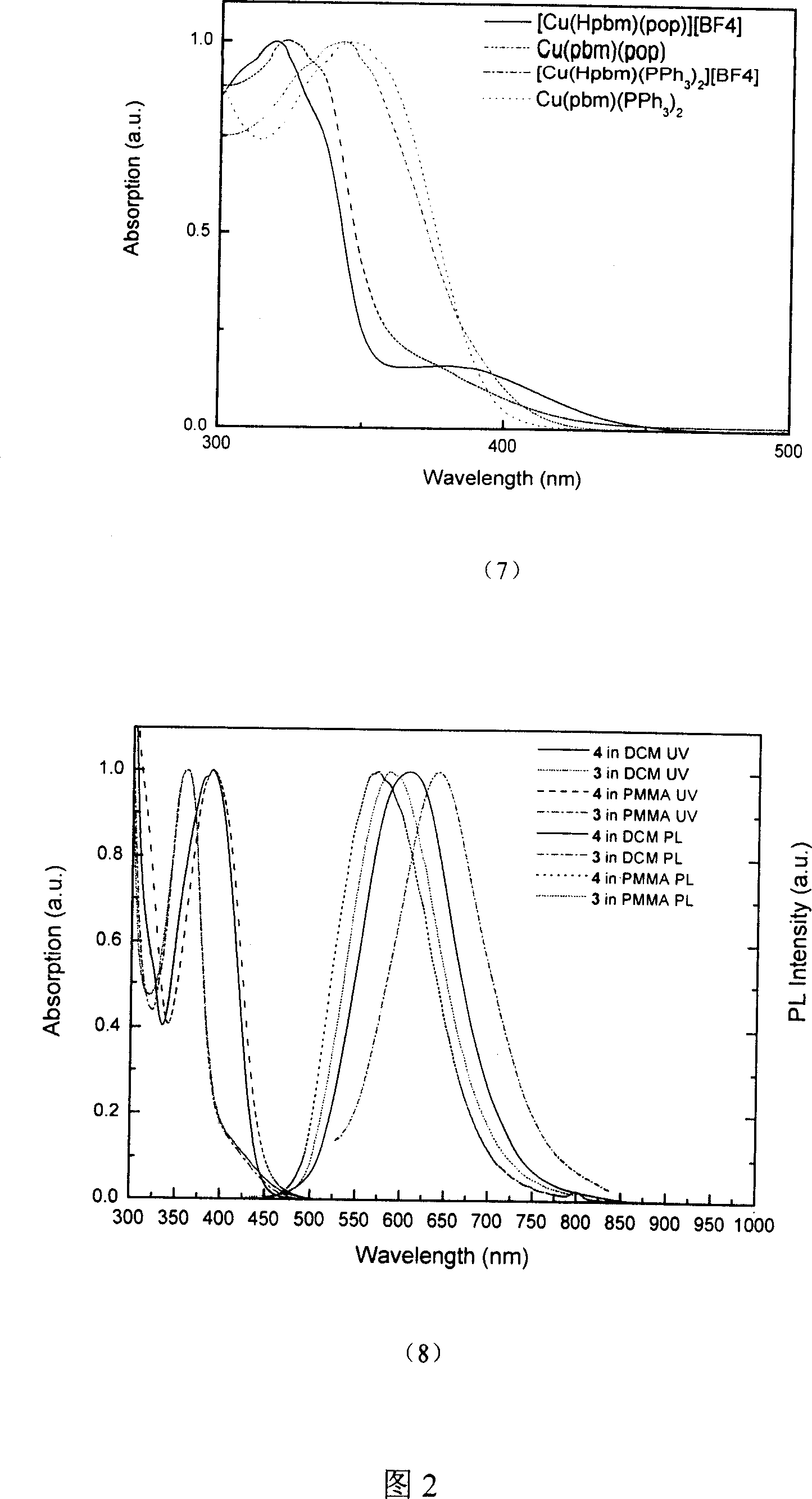
Image
Examples
Embodiment 1
[0061] Embodiment 1[Cu(Hpm)(POP)][BF 4 ] and the synthesis of Cu(pm)(POP)
[0062] 2-(2'-pyridyl)imidazole (Hpm) (145mg, 1.0mmol) was mixed with 2,2'-bis(diphenylphosphino)diphenyl ether (POP) (538mg, 1.0mmol) and [Cu( CH 3 EN) 4 ][BF 4 ] (314mg, 1.0mmol) were co-dissolved in 10mL of dichloromethane to obtain a light green transparent solution. After stirring at room temperature for 5 hours, an appropriate amount of methanol was added for recrystallization to obtain green needle-like crystals [Cu(Hpm)(POP)][BF 4 ] 810mg, yield 86%. 1HNMR (CDCl 3 , 300MHz): δ12.05(s, 1H), 8.28(d, 1H, J=8.1), 8.03(d, 1H), 7.90(t, 1H, J=7.11), 7.34-6.97(m, 29H) , 6.86 (m, 2H).
[0063] [Cu(Hpm)(pop)][BF 4 ] (275 mg, 0.33 mmol) was first dissolved in 5 mL of dichloromethane. Then, it was added to a solution of NaOH (0.53 g, 13.2 mmol) in 100 mL of methanol. After stirring for 10 hours, the solvent was drawn off to give a sticky mass. Extract with 5mL of dichloromethane, filter to obtai...
Embodiment 2
[0064] Embodiment 2 [Cu (Hpm) (PPh 3 ) 2 ][BF 4 ]Synthesis
[0065] 2-(2'-pyridyl) imidazole (Hpm) (145mg, 1.0mmol) and triphenylphosphine (PPh 3 ) (524mg, 2.0mmol) and [Cu(CH 3 EN) 4 ][BF 4 ] (314mg, 1.0mmol) were co-dissolved in 10mL of dichloromethane to obtain a light green transparent solution. After stirring at room temperature for 5 hours, an appropriate amount of methanol was added for recrystallization to obtain green granular crystals [Cu(Hpm)(PPh 3 ) 2 ][BF 4 ] 750mg, yield 76%. 1 HNMR (CDCl 3 , 300MHz): δ12.07(s, 1H), 8.24(d, 1H, J=8.10), 8.08(s, 1H), 7.89(t, 1H, J=7.50), 7.51(s, 1H), 7.41 -7.22 (m, 30H), 6.99 (s, 2H).
Embodiment 3
[0066] Embodiment 3 [Cu (Hpbm) (POP)] [BF 4 ] and the synthesis of Cu(pbm)(POP)
[0067] 2-(2'-pyridyl)benzimidazole (Hpbm) (195mg, 1.0mmol) was mixed with 2,2'-bis(diphenylphosphino)diphenyl ether (POP) (538mg, 1.0mmol) and four Tetrakis(acetonitrile)copper(I)[Cu(CH 3 EN) 4 ][BF 4 ] (314mg, 1.0mmol) were dissolved in 10mL of dichloromethane, and stirred to obtain a grass-green transparent solution. After stirring at room temperature for 1 hour, 10 mL of ether was added for recrystallization to obtain yellow-green needle crystals [Cu(Hpbm)(POP)][BF 4 ] 680mg, yield 77%. 1 HNMR (CDCl 3 , 300MHz): δ12.15(s, 1H), 8.47(d, 1H, J=8.9), 8.15(d, 1H, J=5.3), 7.90(t, 1H, J=8.9), 7.84(d, 1H, J=9.3Hz), 7.28-7.22(m, 7H), 7.12-7.04(m, 15H), 7.01-6.86(m, 10H).
[0068] [Cu(Hpbm)(POP)][BF 4 ] (295 mg, 0.3 mmol) was dissolved in 5 mL of dichloromethane and added to a solution of NaOH (530 mg, 13.2 mmol) in 100 mL of methanol. After stirring for 10 hours, the solvent was pumped dry. ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com