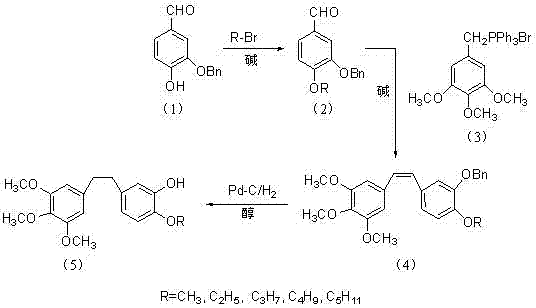
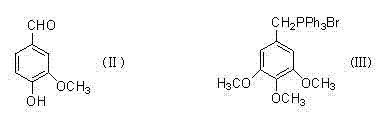Method for preparing 3,4,5-trimethoxy-3'-hydroxy-4'-alkoxy diphenylethane
A technology of alkoxydiphenylethane and trimethoxy, which is applied in the field of synthesis of alkoxydiphenylethane derivatives, and achieves the effects of simple operation method, remarkable technological progress and easy control of synthesis conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] Example 1 compound 2 That is, the preparation of 3-benzyloxy-4-methoxybenzaldehyde
[0035] A dry 500ml oblique three-necked flask with a condenser, mechanically stirred, put in 19.85g (87.06mmol) of the compound 1 , dissolved in 100ml of acetonitrile, heated to 40°C in an oil bath, and added 9.33g (88.04mmol) of Na 2 CO 3 , continue to heat up to 60° C., add 8 ml of methyl bromide, continue to add 8 ml of methyl bromide after 3 hours, and react overnight.
[0036] After the reaction, the system was cooled to room temperature and quenched with 100ml of water, extracted with ethyl acetate, washed twice with saturated brine, dried over anhydrous sodium sulfate, filtered, concentrated and dried, and washed twice with petroleum ether to obtain 20 grams of a white solid. The yield is 95%.
[0037] 1 H NMR (CDCl 3 ) δ(ppm): 9.82 (s, 1H), 7.32-7.46 (m, 7H), 7.00(d, 1H), 5.20 (s, 2H), 3.97 (s, 3H).
Embodiment 2
[0038] Example 2 compound 2 That is, the preparation of 3-benzyloxy-4-ethoxybenzaldehyde
[0039] A dry 500ml oblique three-necked flask with a condenser, mechanically stirred, put in 19.85g (87.06mmol) of the compound 1 , dissolved in 100ml of isopropanol, heated in an oil bath to 40°C, and added 12.15g (88.04mmol) of K 2 CO 3 , continue to heat up to 60°C, add 10ml of bromoethane, continue to add 10ml of bromoethane after 3 hours, continue to react for 3 hours, after TLC detects that the reaction is complete, the system cools down to room temperature, quenches the reaction with 100ml of water, and extracts with ethyl acetate Finally, it was washed twice with saturated brine, dried over anhydrous sodium sulfate, filtered, concentrated and dried, and washed twice with petroleum ether to obtain 19.8 g of a white solid with a yield of 89%.
[0040] 1 H NMR (CDCl 3 ) δ(ppm): 9.81 (s, 1H), 6.98-7.47 (m, 8H), 5.20 (s, 2H), 4.18-4.22 (q, 2H), 1.49-1.52 (t, 3H).
Embodiment 3
[0041] Example 3 Compound 2 That is, the preparation of 3-benzyloxy-4-propoxybenzaldehyde
[0042] A dry 500ml oblique three-necked flask with a condenser, mechanically stirred, put in 19.85g (87.06mmol) of the compound 1 , dissolved in 100ml of DMF, heated to 60°C in an oil bath, added 4.93g (88.04mmol) of KOH, continued to heat up to 80°C, added 12ml of bromopropane, continued to add 12ml of bromopropane after 3 hours, and reacted overnight.
[0043] After the reaction, the system was cooled to room temperature and quenched with 100ml of water, extracted with ethyl acetate, washed twice with saturated brine, dried over anhydrous sodium sulfate, filtered, concentrated and dried, washed twice with petroleum ether to obtain 18.8 grams of a white solid. The rate is 80%.
[0044] 1 H NMR (CDCl 3 ) δ(ppm): 9.85(s, 1H), 7.20-7.48 (m, 8H), 5.20(s, 2H), 3.90 (t, 2H), 1.79 (m, 2H), 0.96 (t, 3H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com