Phenyl phosphine diamide derivative as well as synthesis method and application thereof
A technology of phenylphosphine diamide and synthesis method, which is applied in the fields of nitrogen flame retardant materials and epoxy resins containing phosphorus, can solve problems such as application restrictions, and achieve the goal of avoiding phase separation, uniform and stable flame retardant effect, and ensuring uniform mixing Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0033] The present invention also relates to a method for synthesizing the above-mentioned phenylphosphine diamide derivatives, comprising the following steps:
[0034] (1) use phenylphosphine diacid chloride and primary amine as reaction raw materials, use tetrahydrofuran or diethyl ether as reaction solvent, and use triethylamine as reaction catalyst;
[0035] (2) the implementation of acylation reaction, primary amine is dissolved in ether or tetrahydrofuran, with triethylamine of equivalent amount as bottom material, slowly drip the ether or the tetrahydrofuran solution containing phenylphosphine dichloride under uniform stirring, The dripping time shall not be less than 2 hours;
[0036] (3) temperature of reaction is controlled below 5 ℃, after dripping finishes, continue stirring at room temperature for more than 5 hours to ensure that the reaction is complete;
[0037] (4) The product is filtered to remove triethylamine hydrochloride, and the solvent is recovered and ...
Embodiment 1
[0046] Synthesis of N,N'-diallyl-P-phenylphosphine diamide:
[0047] 0.1 mol of allylamine and 0.105 mol of triethylamine were dissolved in 100 mL of ether, placed in a 250 mL three-necked flask and stirred evenly; the ether solution of phenylphosphine dichloride (0.05 mol of phenylphosphine dichloride) was added dropwise to the flask under an ice-water bath. The acid chloride was dissolved in 50 mL of ether), and the dropwise addition time was 2 hours. After the dropwise addition was completed, stirring was continued at room temperature for 5 hours to complete the reaction. After the reaction was completed, the triethanolamine hydrochloride in the suspension was filtered off by suction filtration, and the filtrate was rotary evaporated to remove diethyl ether to obtain a crude product with a product yield of 94%. The product was washed 3 times with deionized water and dried for later use.
[0048] The purified product was tested and analyzed by the infrared spectrum of the ...
Embodiment 2
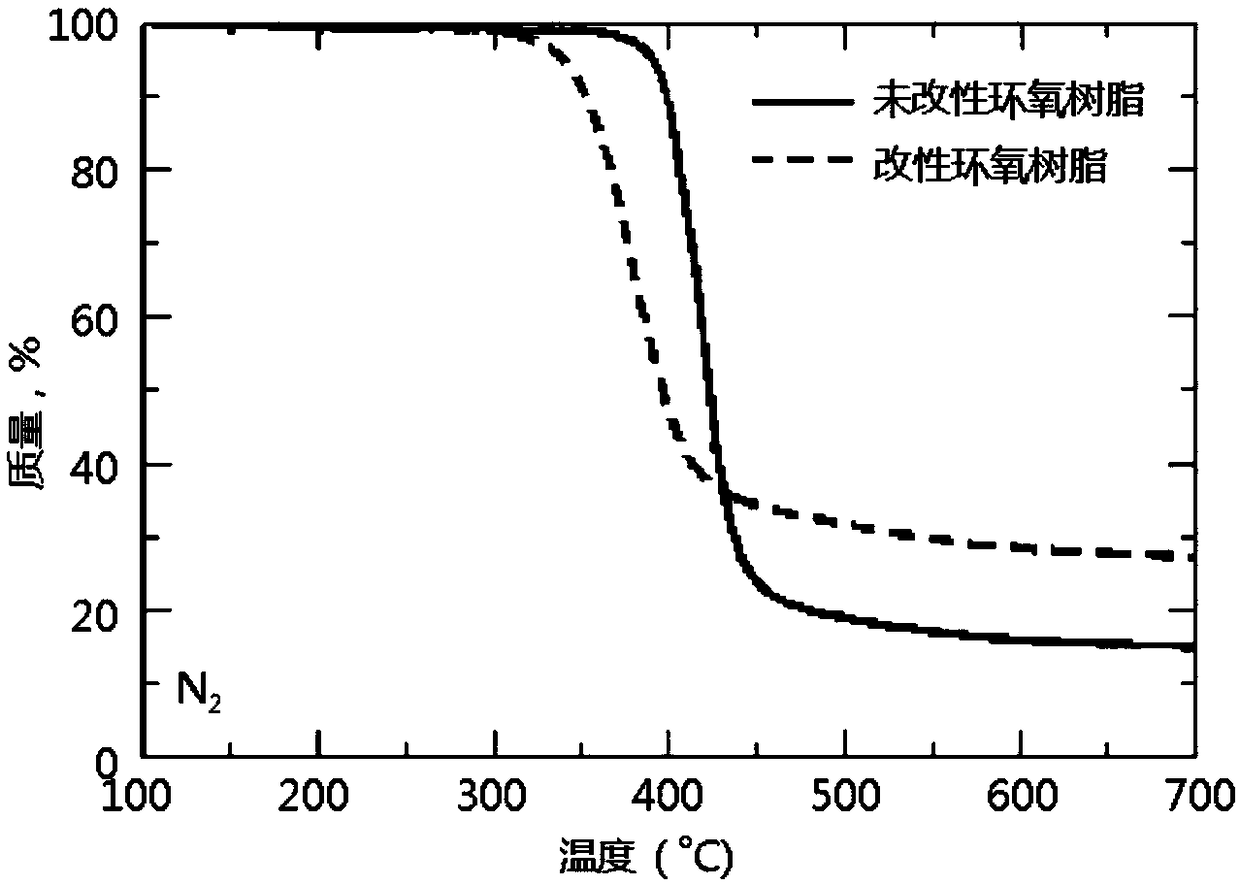
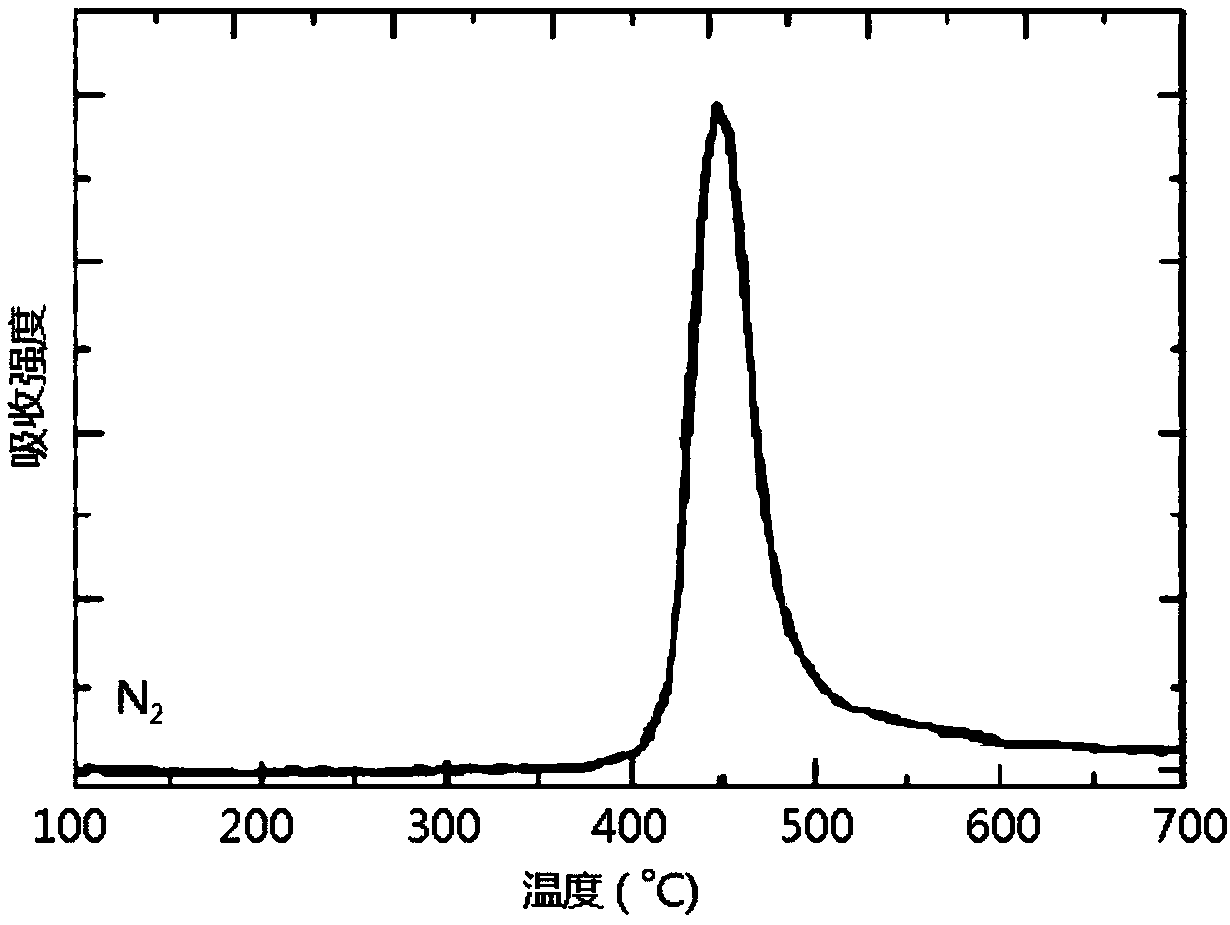
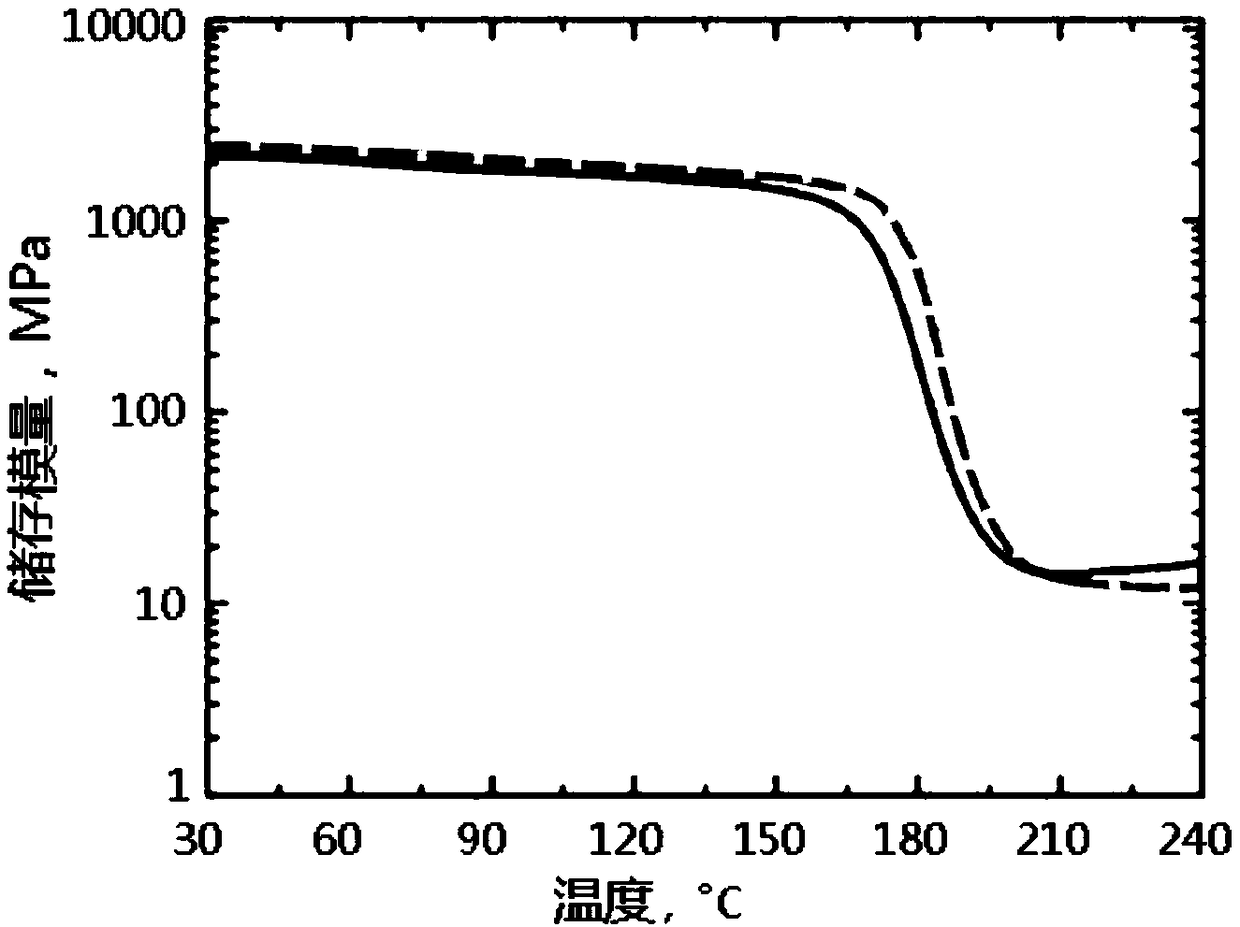
[0053] Flame Retardant Modification of Epoxy Resin Curing System by Phenylphosphine Diamide Derivatives:
[0054] Stir 100g of epoxy prepolymer with epoxy equivalent of 0.54 and 1-5g of phenylphosphine diamide derivative at 100°C for 5 minutes to mix thoroughly; add 33g of diaminodiphenylsulfone, and heat up to 130 ℃ Continue stirring until diaminodiphenyl sulfone is completely dissolved; then add the mixture into a preheated PTFE mold and cure at 160 ℃ for 1 hour, 180 ℃ for 2 hours, and 200 ℃ for 2 hours ; And prepare epoxy curable resin without adding phenylphosphine diamide derivative as blank sample under the same conditions.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com