Reaction-type halogen-free flame retardant bis-(p-aminocarboxyphenyl)phenylphosphine oxide and synthetic method thereof
A technology of aminocarboxyphenyl and phenylphosphine oxide, which is applied in the chemical field, can solve the problems of long cycle time, complex process, and environmental pollution, and achieve the effect of fewer production steps, simple process, and low cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] The synthetic method of reactive type halogen-free flame retardant bis-(p-aminocarboxyphenyl) phenyl phosphine oxide is carried out in the following steps:
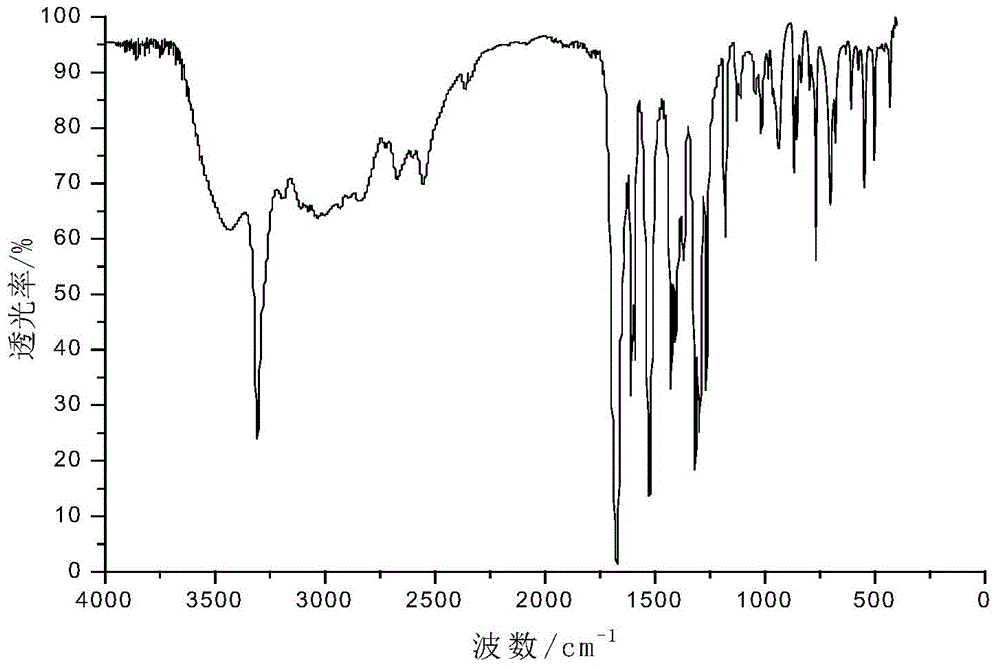
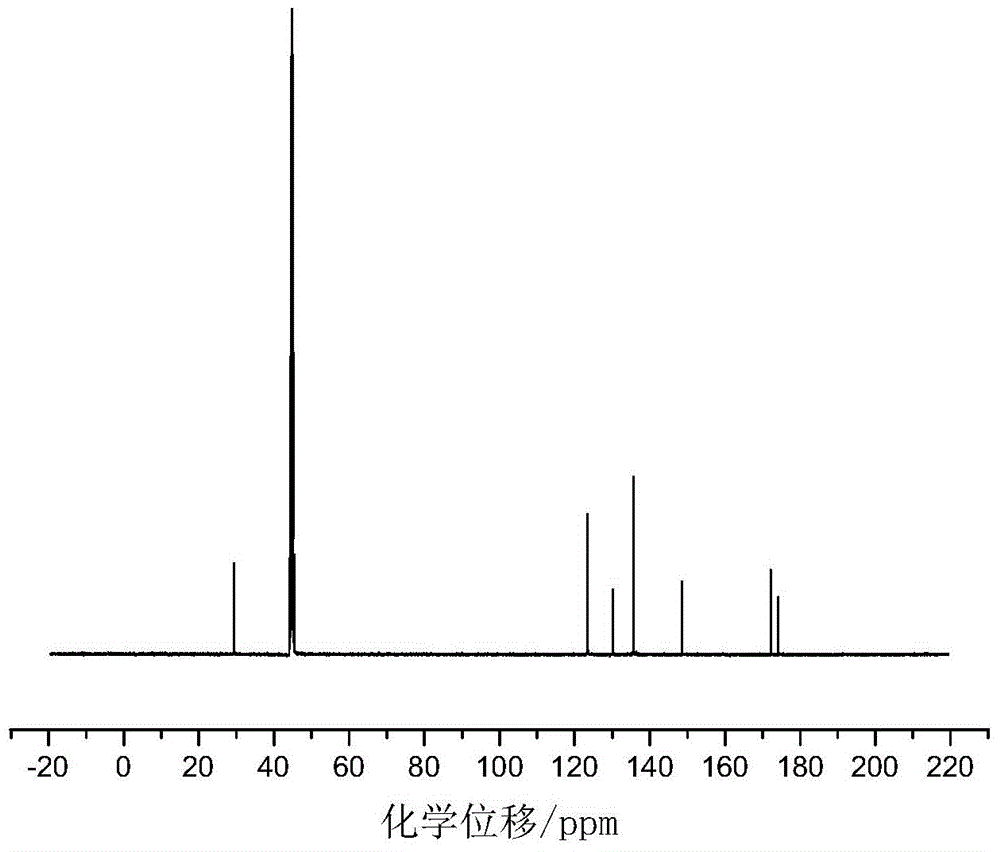
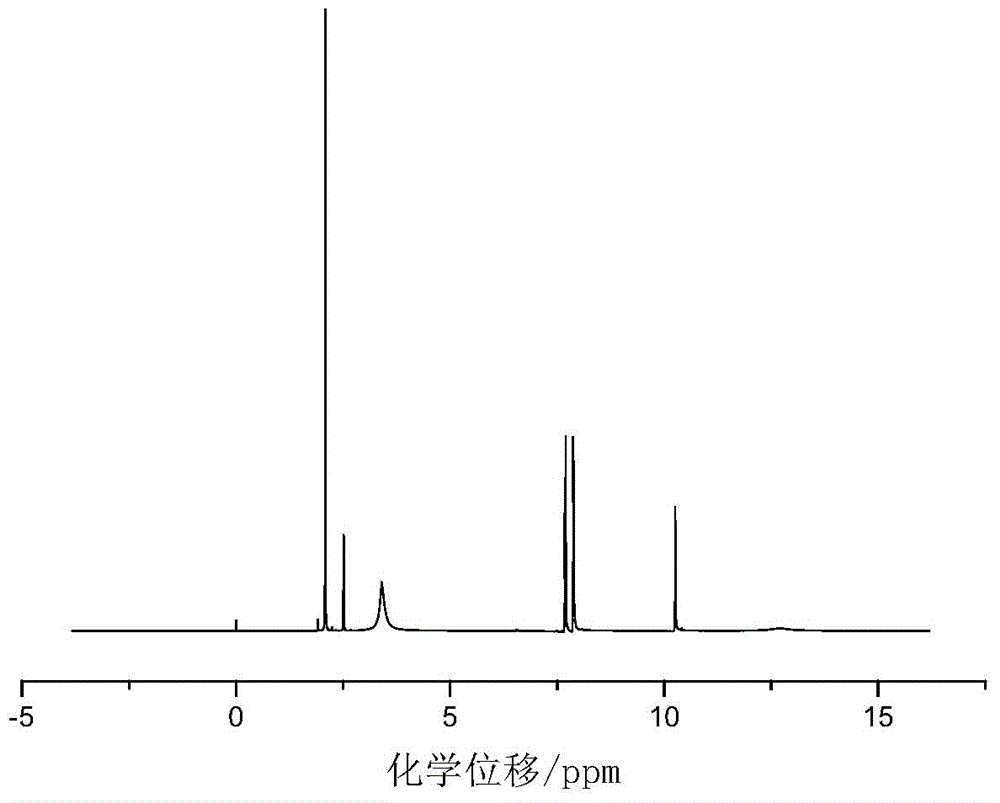
[0028] Add 0.055mol of p-aminobenzoic acid and 30mL of glacial acetic acid into a dry three-necked flask with a capacity of 100mL equipped with a condenser tube, a thermometer and a stirrer, and then slowly add 0.025mol of benzene dropwise under the condition of a stirring speed of 500r / min. Phosphoryl dichloride was added dropwise within 20 minutes, heated to 90°C after addition, refluxed for 5 hours, then cooled to room temperature, then filtered, recovered solvent acetic acid, washed with acetic acid and distilled water in turn, and then used Recrystallize with distilled water, and finally dry at 105°C in a circulating blast drying oven to obtain a white powdery solid with a yield of 84%. The product confirmed by infrared spectroscopy is bis-(p-aminocarboxyphenyl)phenyl Phosphine oxide.
[0029] in figure 1 Th...
Embodiment 2
[0039] The synthetic method of reactive type halogen-free flame retardant bis-(p-aminocarboxyphenyl) phenyl phosphine oxide is carried out in the following steps:
[0040] Add 0.11mol of p-aminobenzoic acid and 60mL of glacial acetic acid into a dry three-neck flask with a capacity of 250mL equipped with a condenser tube, a thermometer and a stirrer, and then slowly add 0.05mol of benzene dropwise at a stirring speed of 500r / min. Phosphoryl dichloride, after the addition, heat up to 85°C, reflux for 6 hours, then cool to room temperature, then filter, recover the solvent acetic acid, then wash with acetic acid and distilled water in turn, and recrystallize with distilled water, after suction filtration Drying with circulating air at 110° C. gave a white powdery solid with a yield of 82%. It was confirmed by infrared spectrum analysis that the obtained product was bis-(p-aminocarboxyphenyl)phenylphosphine oxide. After the reaction product is salted with hexamethylenediamine, it...
Embodiment 3
[0042] The synthetic method of reactive type halogen-free flame retardant bis-(p-aminocarboxyphenyl) phenyl phosphine oxide is carried out in the following steps:
[0043] Add 0.24mol of p-aminobenzoic acid and 120mL of glacial acetic acid into a dry three-neck flask with a capacity of 250mL equipped with a condenser tube, a thermometer, and a stirrer, and then slowly add 0.1mol of benzene dropwise at a stirring speed of 500r / min. Phosphoryl dichloride, after the addition, heat up to 95°C, reflux for 4 hours, then cool to room temperature, then filter, recover the solvent acetic acid, then wash with acetic acid and distilled water in turn, then recrystallize with distilled water, and finally recycle Dried at 106° C. in a blast drying oven to obtain a white powdery solid with a yield of 80%, which was confirmed to be bis-(p-aminocarboxyphenyl)phenylphosphine oxide by infrared spectrum analysis. After the reaction product is salted with hexamethylenediamine, it is copolymerized ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com