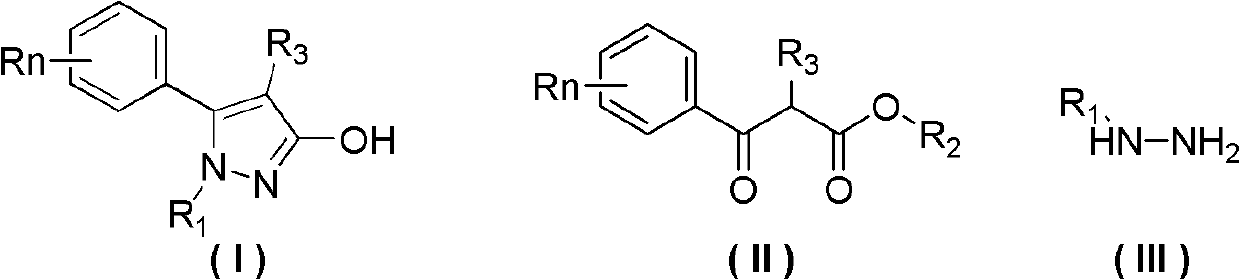
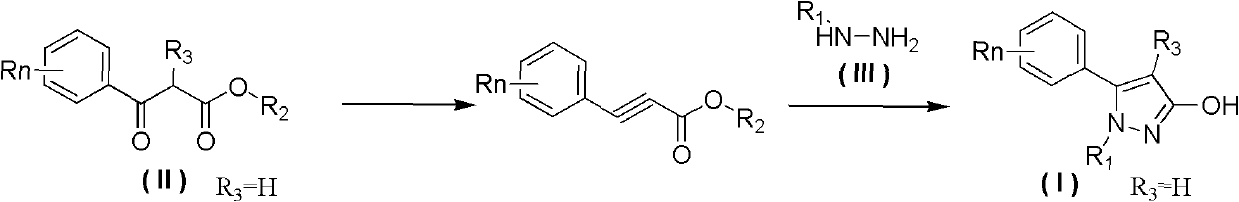
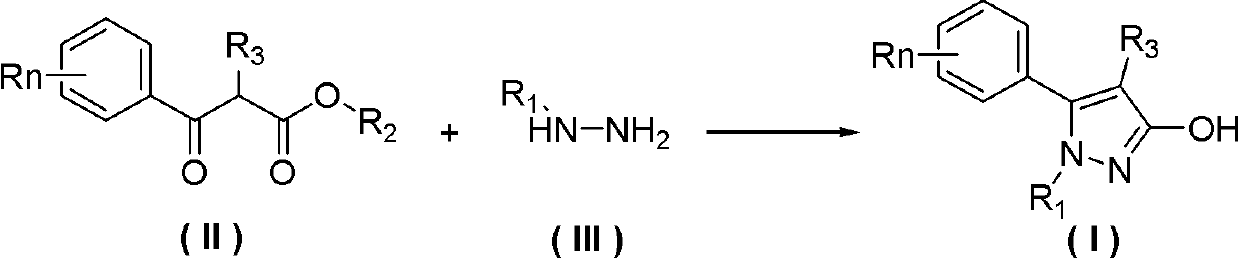Preparation method of 3-hydroxy-substituted pyrazol
A hydroxyl and pyrazole technology is applied in the field of preparation of 3-hydroxy-substituted pyrazoles, and can solve the problems of troublesome separation process of 3-hydroxy-substituted pyrazole products, low reaction selectivity of 3-hydroxy-substituted pyrazoles, and the like, To achieve the effect of easy availability of raw materials, less by-products and simple operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0035] Example 1: Preparation of 2-phenyl-1,3-dimethyl-pyrazole-1H-3-ol (compound 35)
[0036]
[0037] Add 30mL of methylhydrazine (95% content) to a 125mL three-necked flask with a thermometer, and control the temperature in an ice bath below 10°C. The ester (refer to Tetrahedron, 2008, 64, 3471-3476 for the synthesis method) was added to the reaction flask (about 10 minutes), and the reaction was stirred at room temperature for about 4 hours after the addition was completed. Liquid chromatographic detection of the raw material 2-methyl-3-carbonylphenylpropionate methyl ester in the reaction liquid has completely reacted. Distilled under reduced pressure at 50°C to recover 28 mL of methylhydrazine solution, and cooled to room temperature to precipitate a pale yellow solid. Add 40mL of water, stir and filter, then wash the filter cake with 40mL of petroleum ether, collect the solid, and dry to obtain 2.82g of 2-phenyl-1,3-dimethyl-pyrazol-3-ol; then wash the washing liqui...
example 2
[0038] Example 2: Preparation of 5-(4-methylphenyl)-1,4-dimethyl-1H-pyrazol-3-ol (compound 37)
[0039]
[0040] Add 40mL of methylhydrazine (95% content) to a 250mL three-necked flask with a thermometer, and control the temperature in an ice bath below 10°C. At this temperature, 5g (22.1mmol) of 3-(4-tolyl)-2 -Methyl-3-carbonylpropionate (refer to Tetrahedron, 2008, 64, 3471-3476 for the synthesis method) was added to methylhydrazine, and stirred at room temperature for about 4 hours. 3-(4-tolyl)-2-methyl-3-carbonyl propionate raw material reacts completely in liquid chromatography detection reaction liquid, follow-up operation is with example 1, obtains 5-(4-tolyl)-1 altogether , 4-Dimethyl-1H-pyrazol-3-ol 3.7g, white solid, content 98.7%, yield 75%, melting point 222-224°C.
example 3
[0041] Example 3: Preparation of 5-(4-methylphenyl)-1,4-dimethyl-1H-pyrazol-3-ol (compound 37)
[0042]
[0043] Add 5g (22.1mmol) of 3-(4-tolyl)-2-methyl-3-carbonylpropionic acid methyl ester, 40mL methylhydrazine (content 40%) aqueous solution and 5g Sodium chloride, during the feeding process, the ice bath temperature was controlled below 10°C, after the feeding was completed, it was reacted at room temperature for 8 hours until the reaction was complete, and solids were continuously precipitated during the reaction process. After the reaction was complete, the filter cake was collected by filtration, washed with 40 mL of petroleum ether, and dried to obtain 2.1 g of the product. The filtrate was extracted with ethyl acetate, dried, rotary evaporated, column chromatography (gradient elution solvent: ethyl acetate: petroleum ether = 1: 3-1) to obtain 0.7g product, a total of 2.8g product was obtained, white solid, content 99.2 %, yield 56%. The melting point is 222-224°...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com