Process for preparing pemetrexed
A technology of pemetrexed and compounds, applied in the field of pemetrexed, can solve the problems of long operation period and complex process, and achieve the effect of simple operation and favorable industrial production
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] Add 100ml of deionized water, 10.0g (68.0mmol) of L-glutamic acid, and 7.0g (69.0mmol) of N-methylmorpholine into the reaction flask, stir until completely dissolved, then add 200ml of dimethylformamide to obtain a clear solution.
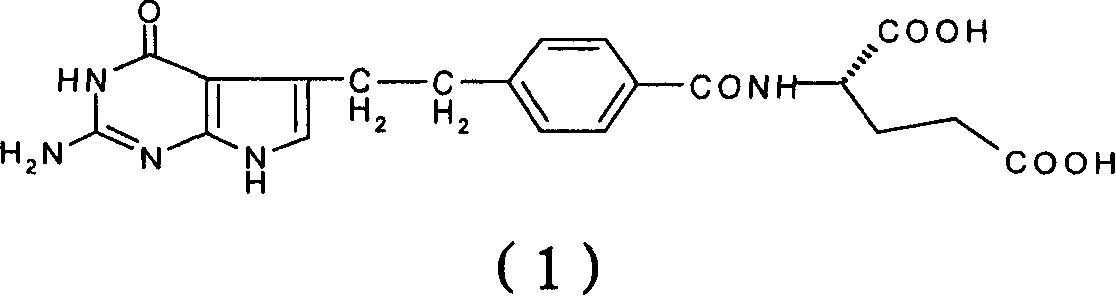
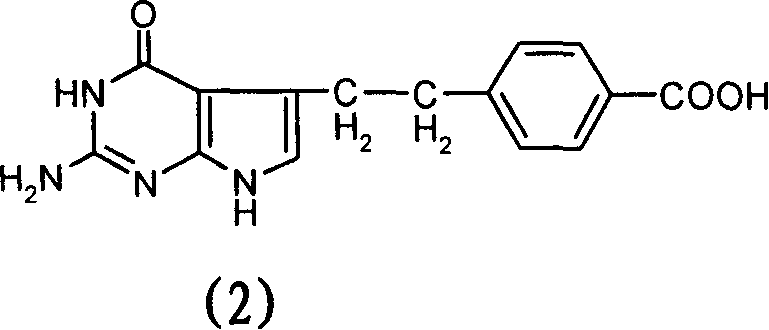
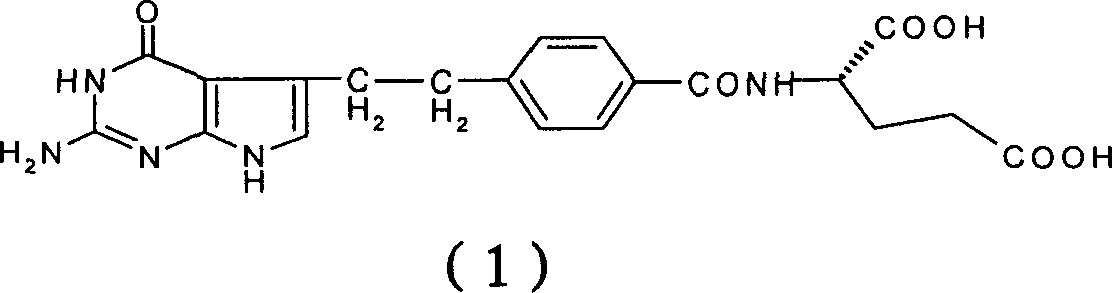
[0031] Under a nitrogen atmosphere, 7.0 g (69.0 mmol) of N-methylmorpholine and 11.7 g (66.6 mmol) of 4-chloro-2,6-dimethoxytriazine were added to 4-[2-(2-amino -4,7-dihydro-4-oxo-3H-pyrrolo[2,3-d]pyrimidin-5-yl)ethyl]benzoic acid (10.0g, 33.5mmol) in dimethylformamide (150ml ) solution, stirred at room temperature for 1 hour. The reaction solution was slowly dropped into the L-glutamic acid solution prepared above in about 0.5 hours to obtain a clear reaction solution, which was stirred at room temperature for 2 hours. Suction filtration, the filtrate was adjusted to pH 2.5-3.5 with 2N hydrochloric acid, the reaction solution was transferred to a 5L reaction flask, 4L deionized water was slowly added, stirred for 1 hour, and suction filtr...
reference example 1
[0034]Under a nitrogen atmosphere, 7.0 g (69.0 mmol) of N-methylmorpholine and 11.7 g (66.6 mmol) of 4-chloro-2,6-dimethoxytriazine were added to 4-[2-(2-amino -4,7-dihydro-4-oxo-3H-pyrrolo[2,3-d]pyrimidin-5-yl)ethyl]benzoic acid (10.0g, 33.5mmol) in dimethylformamide (200ml ) solution. Stir at room temperature for 1 hour, add 7.0 g (69.0 mmol) of N-methylmorpholine and 10.0 g (41.7 mmol) of L-diethyl glutamate hydrochloride, and stir at room temperature for 2 hours. Add 400ml of deionized water and 200ml of dichloromethane to the reaction solution, wash the organic layer with 200ml of deionized water × 2, concentrate, purify by silica gel chromatography (eluent, methanol: dichloromethane 1: 4), combine the pure components, Concentration to give the product N-[4-[2-(2-amino-4,7-dihydro-4-oxo-3H-pyrrolo[2,3-d]pyrimidin-5-yl)ethyl]benzene Formyl]-L-glutamic acid diethyl ester 6.7g (13.9mmol), yield 41.5%.
[0035] The above product N-[4-[2-(2-amino-4,7-dihydro-4-oxo-3H-pyrrol...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com