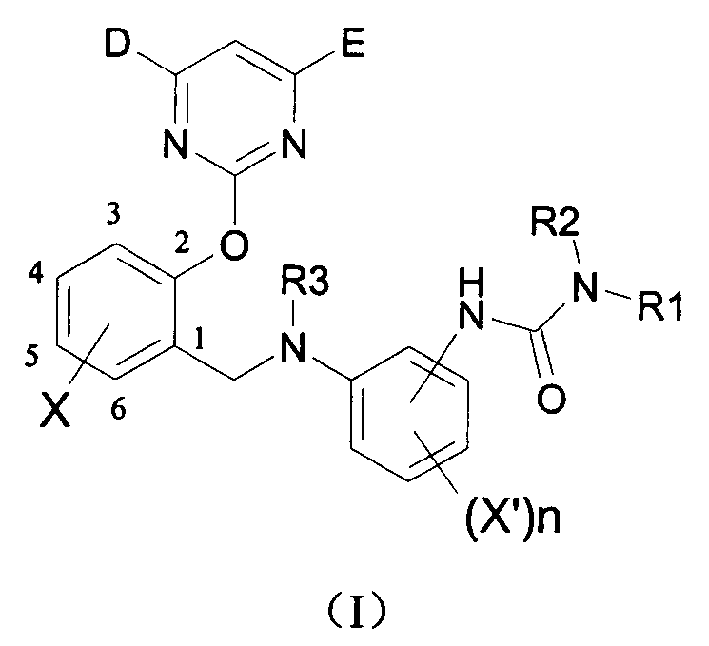
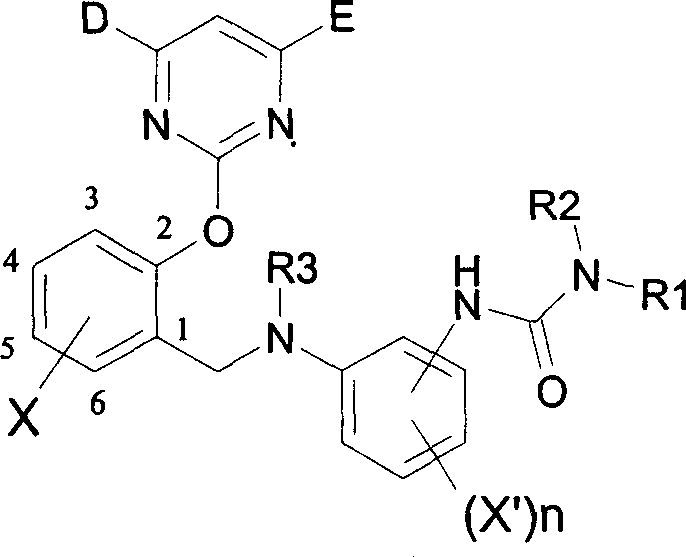
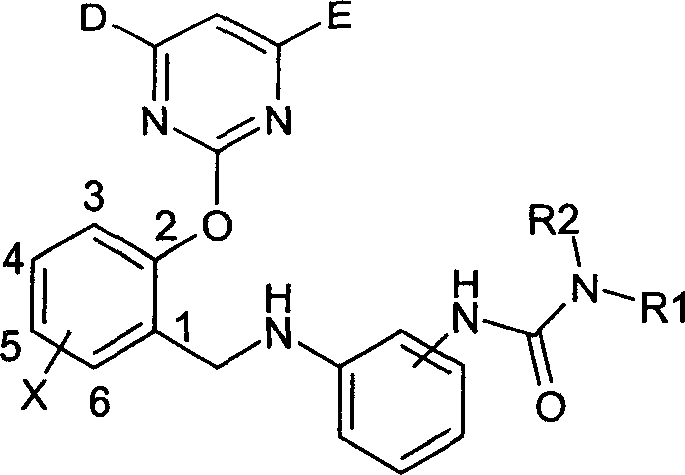
2-pyrimidine oxy-n-ureido phenyl-benzyl amide compound, preparing method and use thereof
A technology of ureidophenylbenzylamine and pyrimidineoxy, which is applied in the field of agrochemical herbicides and can solve problems such as limited varieties of new pesticides
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0051] 1. Synthesis of intermediate (II)
[0052] (a) Synthesis of intermediate (II-1)
[0053] The first step: Take the synthesis of 1-p-nitrophenyl-3-diethylurea as an example: under the protection of argon, add 0.82 grams (5 mmol) of para Dissolve nitrophenyl isocyanate and 5 mL of anhydrous benzene, 0.57 mL (5.5 mmol) of diethylamine in 5 mL of anhydrous benzene, cool to 0-10 ° C, add dropwise to the reaction solution, stir for about 30 minutes after the addition, and spin dry , The pure product was obtained by column chromatography with a yield of 98%.
[0054] The second step: Nitro reduction: Dissolve 5 mmol 1-p-nitrophenyl-3-diethylurea in 20 mL of anhydrous methanol, add 90 mg (equivalent to 1-p-nitrophenyl-3-diethylurea The mass of 7.5%) Raney Ni, 0.469 g (7.5 mmol) of 80% hydrazine hydrate was added dropwise to the reaction solution, stirred at room temperature until the reaction was complete, and the end of the reaction was controlled by TLC. After filtration, t...
Embodiment 2
[0074] The synthesis of I-2, the detailed experimental steps are the same as in Example 1: (1) Condensation with salicylaldehyde: feeding amount 5.21mmol1-m-aminophenyl-3-diethylurea, filtered to obtain 1.620 grams of imines, yield 99%. (2) Schiff base reduction: the amount of imine charged was 5.21 mmol, and 1.630 g (5.21 mmol) of the product was obtained, with a yield of 99%. (3) Condensation with pyrimidine sulfone: 1.630 g (5.21 mmol) of the imine-reduced product was obtained by column chromatography (petroleum ether: ethyl acetate = 2:1) to obtain a pure product with a yield of 85%.
[0075] solid
[0076] m.p.: 100.4±0.5℃
[0077] 1 H NMR (300MHz, CDCl 3 / TMS): δ1.21 (6H, t, CH 3 , J=7.14Hz), 3.36 (4H, q, CH 2 , J=7.14Hz), 3.80 (6H, s, OCH 3 ), 4.34 (2H, s, CH 2 ,), 5.77 (1H, s, CH), 6.21-7.47 (8H, m, CH) MS (EI): 45 (m / z, 100), 452 (M+1, 2.01)
[0078] IR (KBr / cm -1 ): 3406 (γ N-H ), 3309 (γ N-H ), 1639 (γ C=O ), 1598, 1539, 1484 (γ C=C), 1572 (γ C=N ), 1...
Embodiment 3
[0081] The synthesis of I-3, the detailed experimental procedure is the same as that of Example 1: (1) Condensation with 6-chlorosalicylaldehyde: feed amount 4mmol amino, spin dry and go directly to the next step, the yield is 99%. (2) Schiff base reduction: the amount of imine was 4 mmol, and 1.39 g (4 mmol) of the product was obtained, with a yield of 99%. (4) Condensation with pyrimidine sulfone: Synthesize by method b, feed amount 1.39 g (4 mmol) imine reduction product, column chromatography (petroleum ether: ethyl acetate=5: 1) to obtain pure product, yield 90% .
[0082] solid
[0083] m.p.: 153.6±0.5℃
[0084] 1 H NMR (300MHz, CDCl 3 / TMS): δ1.21 (6H, t, CH 3 , J=6.9Hz), 3.35 (4H, q, CH 2 , J=6.9Hz), 3.78 (6H, s, OCH 3 ), 4.43 (2H, d, CH 2 , J=2.7Hz), 5.75 (1H, s, CH), 6.17-7.30 (7H, m, CH)
[0085] MS (EI): 157 (m / z, 100), 485 (M + , 3.97)
[0086] IR (KBr / cm -1 ): 3418 (γ N-H ), 3300 (γ N-H ), 1637 (γ C=O ), 1607, 1568, 1541 (γ C=C ), 1694 (γ C=N ), ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com