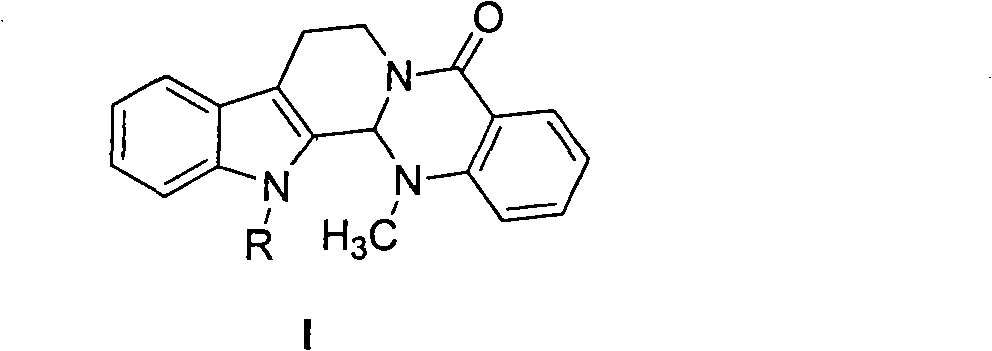
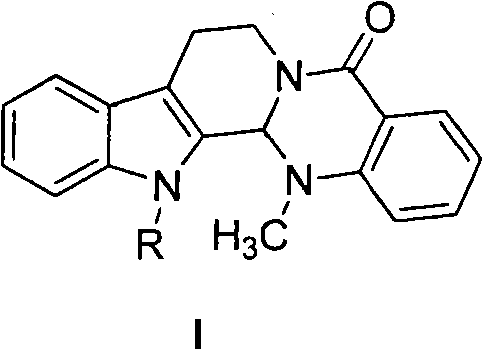
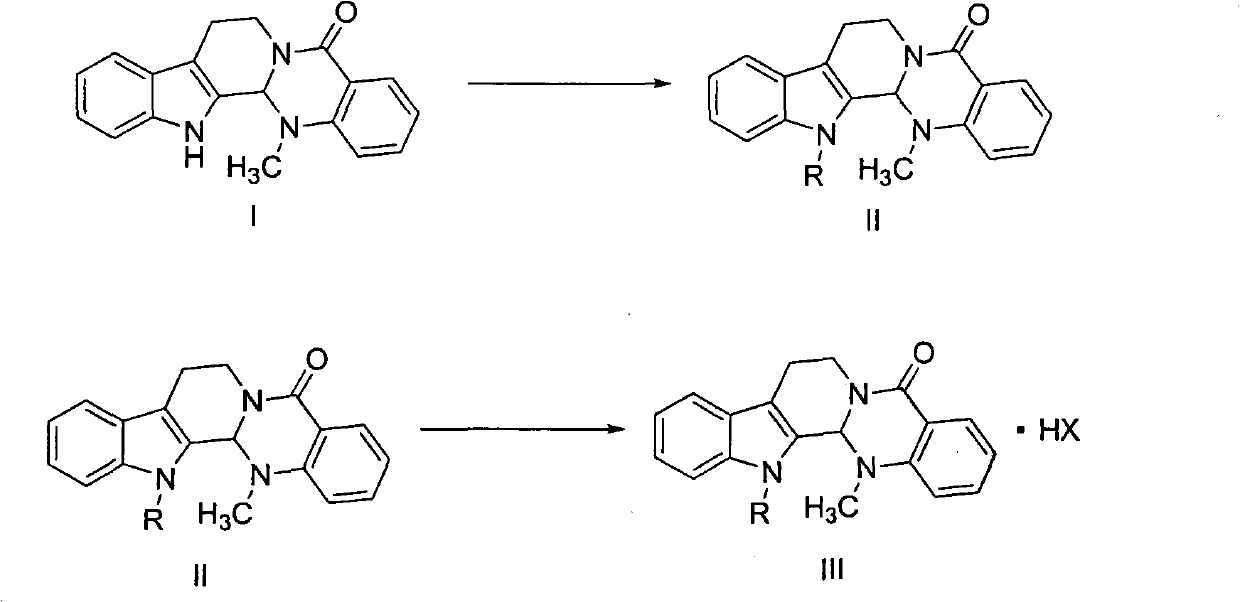
Substituted-evodiamine anti-tumor and antifungal compounds and preparation method thereof
A technology for evodiamine and compounds, which is applied in the field of substituted evodiamine compounds and their salts and their preparation, can solve problems such as weak enzyme inhibitory activity and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] Example 1: Preparation of 3-bromopropyl substituted evodiamine compounds (compound 1 in Table 1)
[0039] Take 0.18g (7.5mmol) of NaH and put it in a 50ml distillation bottle, add 15ml of anhydrous DMF and stir at room temperature for 10 minutes, add 0.30g (1mmol) of evodiamine and continue stirring for 30 minutes at room temperature, then add 1,3-di Propane bromide 0.8g (4mmol), slowly warm up to 80°C and react for 24 hours. Stop the reaction, add 40mlH to the reaction solution 2 O, extract with 40ml×3 ethyl acetate, combine the ethyl acetate extracts, dry with an appropriate amount of anhydrous sodium sulfate, filter, and take the filtrate. The solvent was evaporated to dryness, and purified by silica gel column chromatography, the eluent was petroleum ether: ethyl acetate = 4:1, and 0.15 g of white solid was obtained with a yield of 35.4%. 1 H-NMR (500MHz, DMSO) δ: 2.27(m, 2H, C17-2H), 2.39(s, 3H, C15-3H), 2.81(m, 1H, C8-H), 2.98(m, 1H, C8 -H), 3.11(m, 1H, C7-H), ...
Embodiment 2
[0040] Example 2: Preparation of evodiamine-like compounds substituted by acetonitrile (compound 3 in Table 1)
[0041] Take 0.18g (7.5mmol) of NaH and put it in a 50ml distillation bottle, add 15ml of anhydrous DMF and stir at room temperature for 10 minutes, add 0.30g (1mmol) evodiamine and continue stirring at room temperature for 30 minutes, then add bromoacetonitrile 1.2g ( 10 mmol), slowly warming up to 80°C for 24 hours. Stop the reaction, add 40mlH to the reaction solution 2 O, extract with 40ml×3 ethyl acetate, combine the ethyl acetate extracts, dry with an appropriate amount of anhydrous sodium sulfate, filter, and take the filtrate. The solvent was evaporated to dryness, and purified by silica gel column chromatography, the eluent was petroleum ether: ethyl acetate = 4:1, and 0.15 g of white solid was obtained with a yield of 43.9%. 1 H-NMR (500MHz, DMSO) δ: 2.39(s, 3H, C15-3H), 2.82(m, 1H, C8-H), 2.98(m, 1H, C8-H), 3.14(m, 1H, C7 -H), 4.66(m, 1H, C7-H), 5.57(s,...
Embodiment 3
[0042] Example 3: Preparation of ethoxycarbonyl-substituted evodiamine compounds (compound 4 in Table 1)
[0043] Take 0.18g (7.5mmol) of NaH and put it in a 50ml distillation bottle, add 15ml of anhydrous DMF and stir at room temperature for 10 minutes, add 0.30g (1mmol) evodiamine and continue stirring at room temperature for 30 minutes, then add ethyl chloroformate 1.1 g (10 mmol), slowly warming up to 80°C for 24 hours. Stop the reaction, add 40mlH to the reaction solution 2 O, extract with 40ml×3 ethyl acetate, combine the ethyl acetate extracts, dry with an appropriate amount of anhydrous sodium sulfate, filter, and take the filtrate. The solvent was evaporated to dryness, and purified by silica gel column chromatography, the eluent was petroleum ether: ethyl acetate = 4:1, and 0.17 g of white solid was obtained with a yield of 45.3%. 1 H-NMR (500MHz, CDCl 3 )δ: 1.29(t, J=6.7Hz, 3H, C18-3H), 2.45(s, 3H, C15-3H), 2.86(m, 1H, C8-H), 2.99(m, 1H, C8- H), 3.20(m, 1H, C7-H...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com