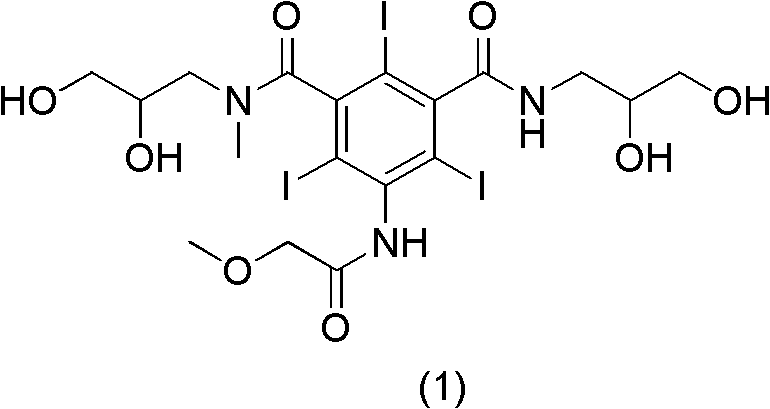
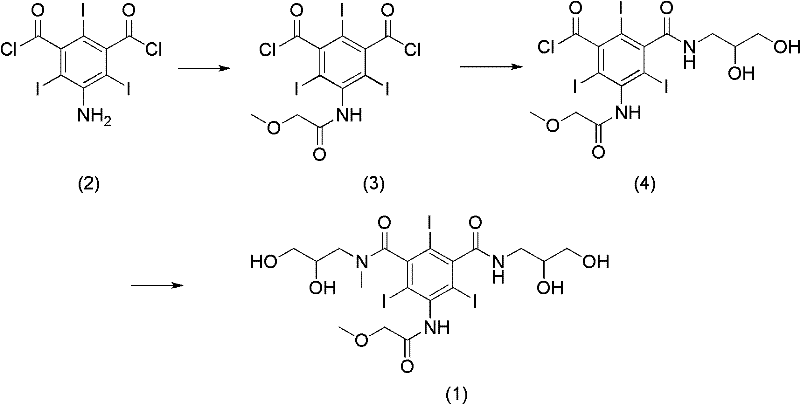
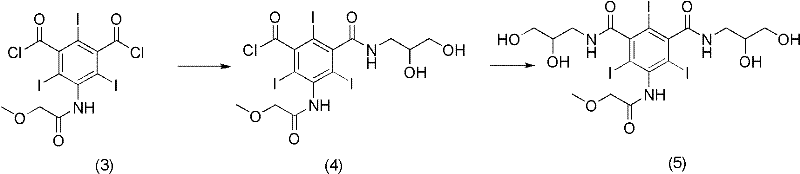
Preparation method of Iopromide
A technology of iopromide and triiodobenzoic acid, which is applied in the field of preparation of iopromide, can solve the problems of increasing iopromide synthesis steps and excessive amount
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0048] Example 1: Synthesis of 3-methoxyacetylamino-5-(2,3-diacetoxy n-propylcarbamoyl)-2,4,6-triiodobenzoic acid (Formula 8)
[0049] In the 2L there-necked flask equipped with a condenser tube, add the compound formula (7) 3-amino-5-(2,3-diacetoxy n-propylcarbamoyl)-2,4,6-triiodobenzoic acid ( 320g, 0.45mol), methoxyacetyl chloride (147g, 1.35mol), DMF (8ml), chloroform (1500ml), reflux reaction for 15 hours, and TLC detected that the reaction was complete. Concentrate under reduced pressure, add ethyl acetate (1500ml), stir at room temperature for 0.5 hours, suction filter, and dry to obtain formula (8) as a white solid compound (345g), with a molar yield of 97.3%.
Embodiment 2
[0050] Embodiment 2: the synthesis of 3-methoxyacetylamino-5-(2,3-dihydroxy n-propylcarbamoyl)-2,4,6-triiodobenzoic acid (formula 9)
[0051] In the 500ml there-necked flask, add compound formula (8) 3-methoxyacetamido-5-(2,3-diacetoxy n-propylcarbamoyl)-2,4,6-triiodobenzoic acid (87g , 0.11mol), sodium bicarbonate (65g, 0.77mol), water (261ml), 55 ℃ insulation reaction for 18 hours, TLC detection reaction is complete. Use 5% hydrochloric acid to adjust pH=5-6, filter with suction, concentrate the filtrate to dryness under reduced pressure, add absolute ethanol (100ml), filter, adjust the filtrate to pH=1-2 with 11% hydrochloric acid, concentrate to dryness under reduced pressure to obtain Formula (9) white solid compound (76.4g), the molar yield is 98.7%.
Embodiment 3
[0052] Embodiment 3: the synthesis of 3-methoxyacetylamino-5-(2,3-dihydroxy n-propylcarbamoyl)-2,4,6-triiodobenzoyl chloride (formula 4)
[0053] In the 1L three-neck flask equipped with a dry condenser, add the compound formula (9) 3-methoxyacetamido-5-(2,3-dihydroxy-n-propylcarbamoyl)-2,4,6-triiodo Benzoic acid (72.5g, 0.10mol) and thionyl chloride (720ml) were incubated at 65°C for 5 hours, and the reaction was completed by TLC. Concentrate under reduced pressure to remove thionyl chloride, add chloroform (150ml), stir at room temperature for 1 hour, filter with suction, and dry in vacuo to obtain formula (4) as a white solid compound (67.9g), with a molar yield of 94%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com