Preparation method of Niraparib
A reagent and solvent technology, applied in the field of preparation of niraparib, can solve the problems of long synthetic route and high cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
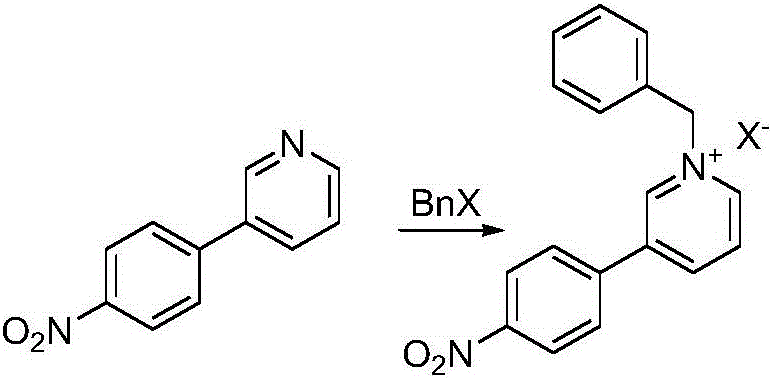
[0044] Example 1 Add 120 mL of benzyl bromide to 30.0 g (0.15 mol) of compound 2, heat the reaction solution to 110 ° C for 10 hours, then cool to room temperature, a solid is precipitated, the solid is filtered out, and washed twice with hexane, and the The obtained solid was dried in a blast oven at 45° C. to obtain 45.2 g of brown color.
[0045]
example 2
[0046] Example 2 Compound 4 was prepared with reference to Example 1
[0047]
example 3
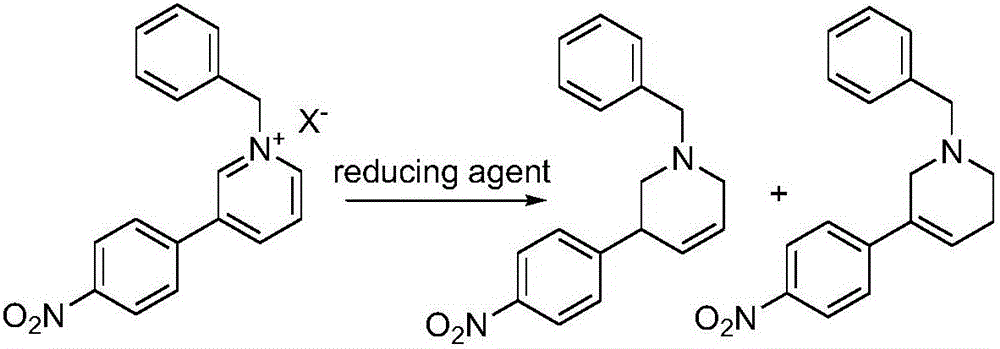
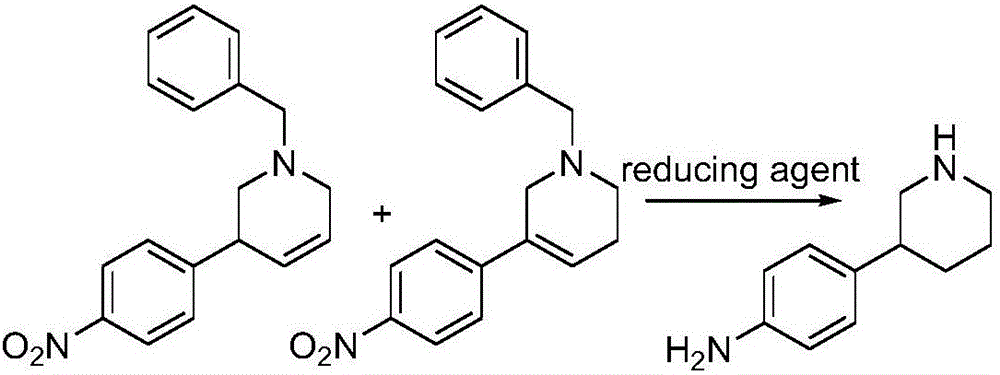
[0048] Example 3 Add compound 3 (43.0g, 0.12mol) to 130mL of methanol, cool the reaction solution to 3-5°C, add sodium borohydride (18.2g, 0.48mol) in partitions, and stir at this temperature after the addition is complete After 30 minutes, rise to room temperature and react for 8 hours. After distilling off most of the methanol under reduced pressure, add 110 mL of water and extract with ethyl acetate twice (2*150 mL), and dry the organic phase with anhydrous sodium sulfate. , the organic phase was steamed under pressure to obtain 22.8 g of light yellow solid.
[0049]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com