Pharmaceutical composition containing a compound having a thrombopoietin receptor agonistic activity
a technology of thrombopoietin and compound, which is applied in the direction of drug composition, extracellular fluid disorder, biocide, etc., can solve the problems of thrombocytopenia patients not being able to receive anti-virus therapy using interferon, bleeding tendency, and reducing the number of platelets, so as to avoid thrombosis risk, increase platelet count, and increase platelet count
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
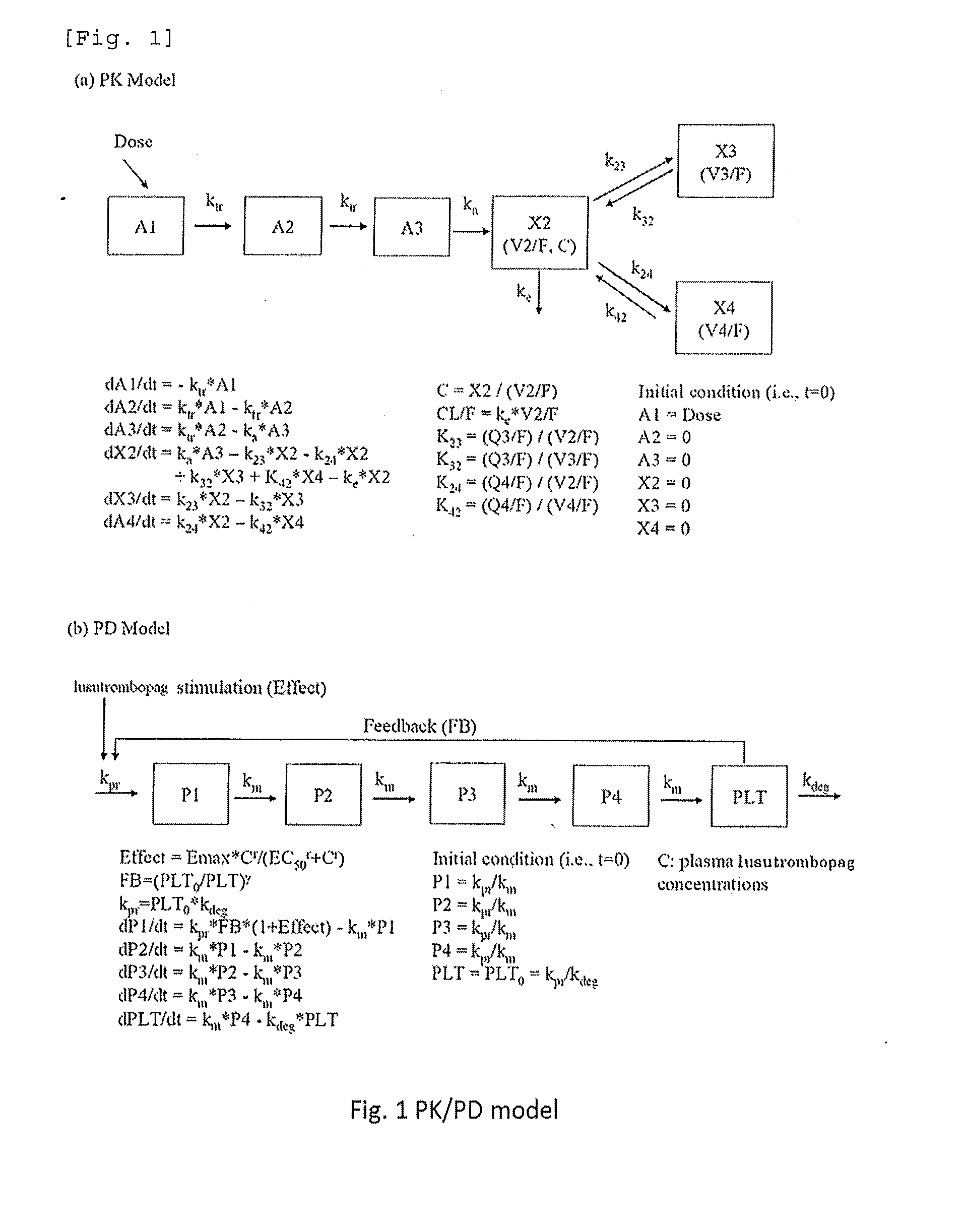
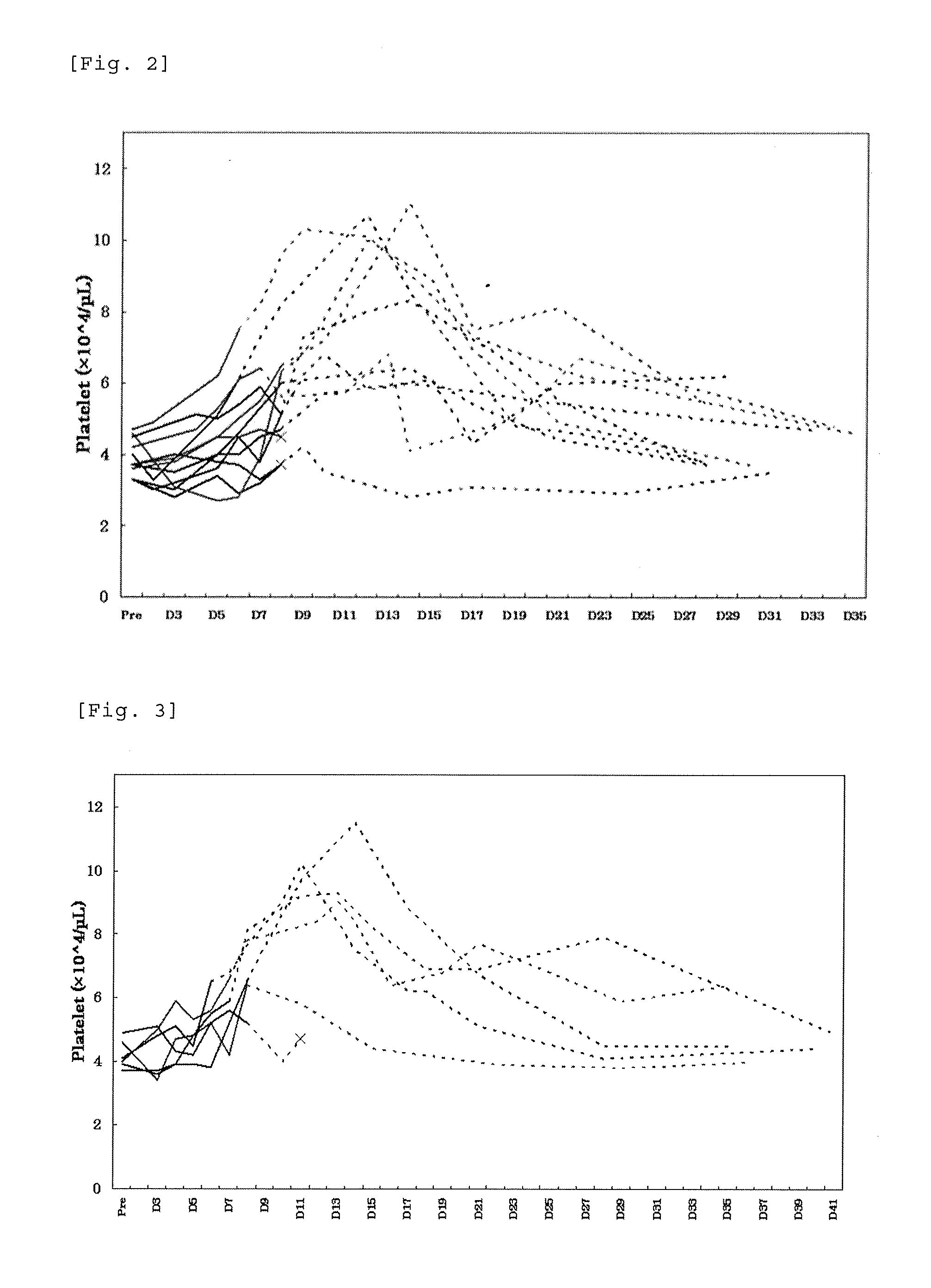
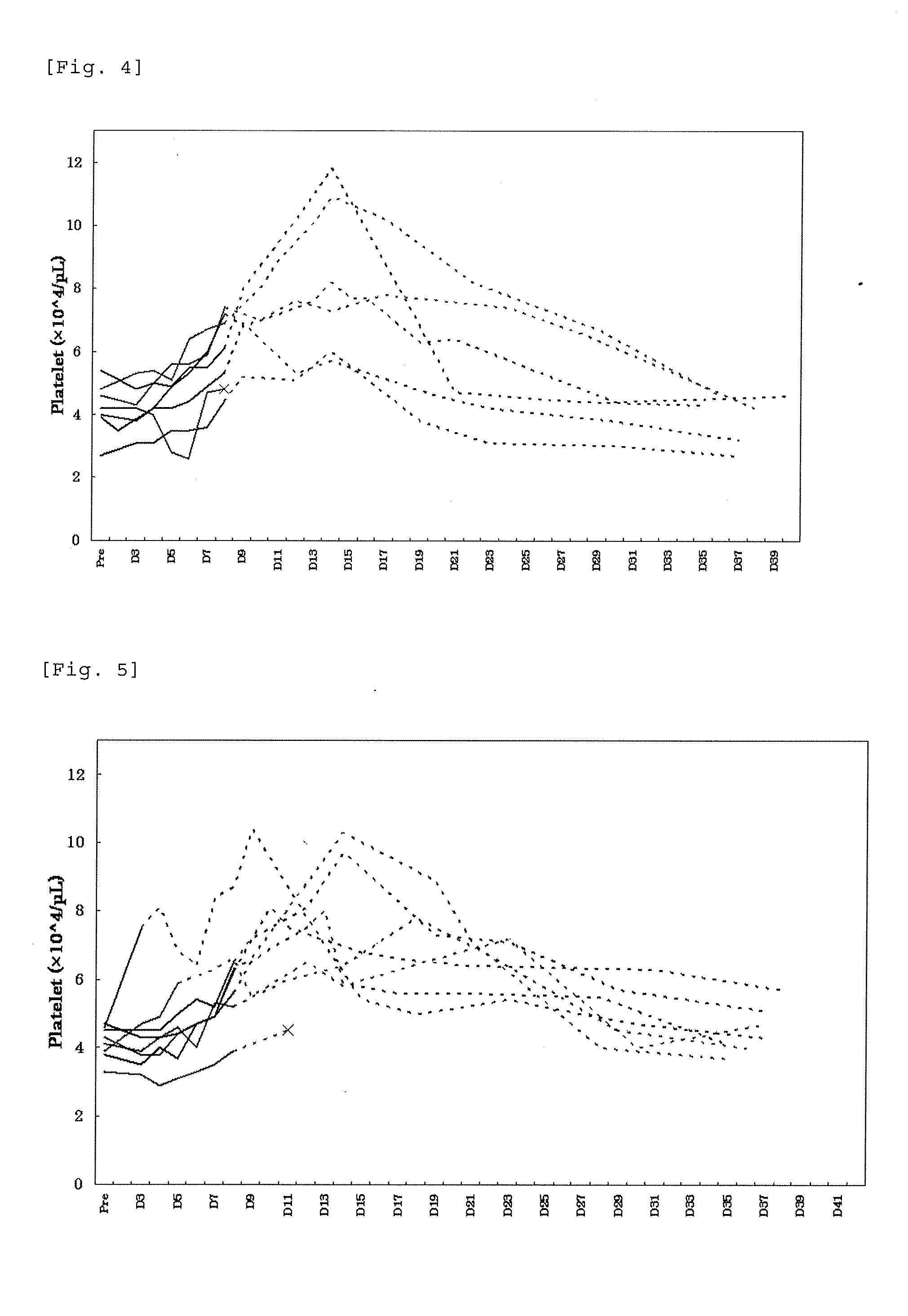
Prediction of Profile of a Platelet Count by Monte-Carlo Simulation
[0216]An excessive increase in the platelet count is considered to increase a risk of thrombosis. In order to ensure the safety of patients who receive drugs, we designed an administration discontinuating criteria based on the platelet count. Using the data of pharmacokinetics and profile of a platelet count from single administration and repeated administration trials of lusutrombopag carried out to healthy adults, a profile of a platelet count during repeated administration of lusutrombopag for 7 days to chronic liver disease patients was predicted by Monte-Carlo simulation. In simulation, the PK / PD model parameters of healthy adults were estimated based on the data obtained from healthy adults (Test Example 1), and model parameters in chronic liver disease patients were calculated based on the PK / PD model in healthy adults (Test Example 2). Based on the calculated parameters, we verified the criteria in which admi...
example 2
Test Example 1
[0256]To patients with thrombocytopenia caused by chronic liver disease, lusutrombopag as a pretreatment of percutaneous liver ablation was orally administered repeatedly for 7 days while applying the discontinuation criteria designed in Example 1 and profile of the platelet count was studied. In the present Example, the term “ablation” refers to radiofrequency ablation.
(Target Disease)
[0257]Patients with thrombocytopenia caused by chronic liver disease who are scheduled to undergo percutaneous liver ablation.
(Inclusion Criteria)
[0258]Patients who meet the following inclusion criteria are included.
1) Age: 20 years of age or older (at the time of signing informed consent);
2) Patients who themselves can give a consent in writing;
3) Patients who have a complication or a history of chronic liver disease caused by hepatitis B or C virus;
4) Patients who are scheduled to undergo percutaneous liver ablation for primary liver cancer;
5) Patients having a platelet count which is ...
formulation examples
[0290]The following Formulation Examples are only exemplified and not intended to limit the scope of the invention.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Time | aaaaa | aaaaa |
| Mass flow rate | aaaaa | aaaaa |
| Composition | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com