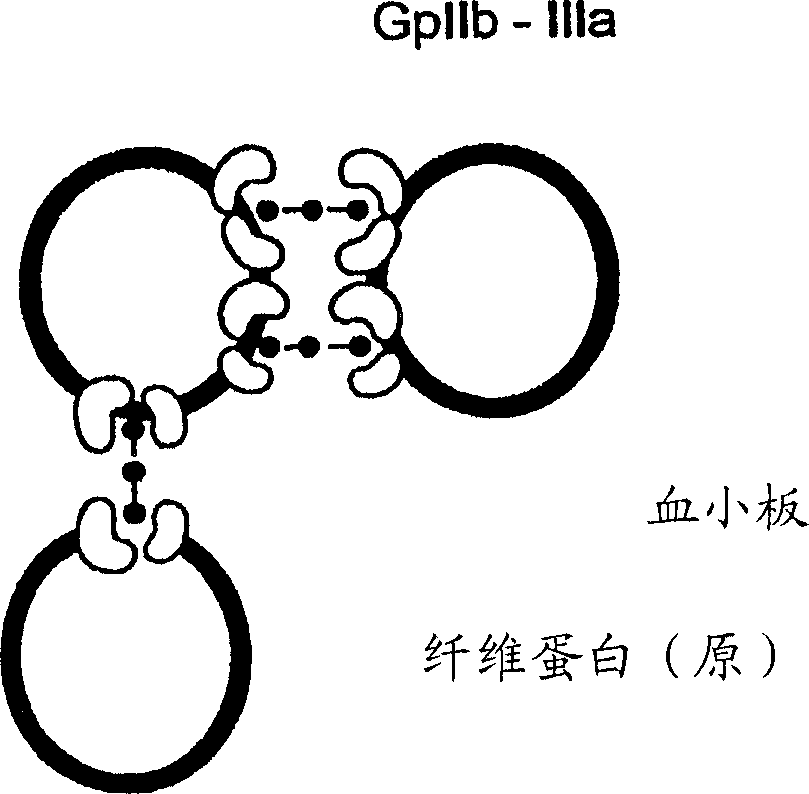
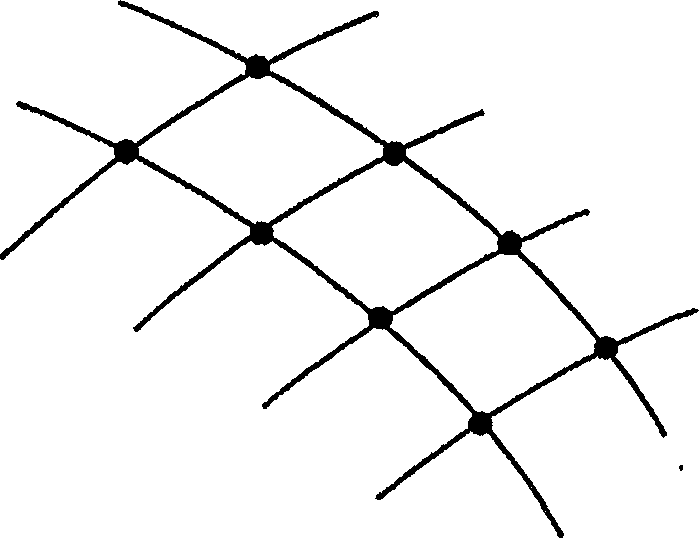
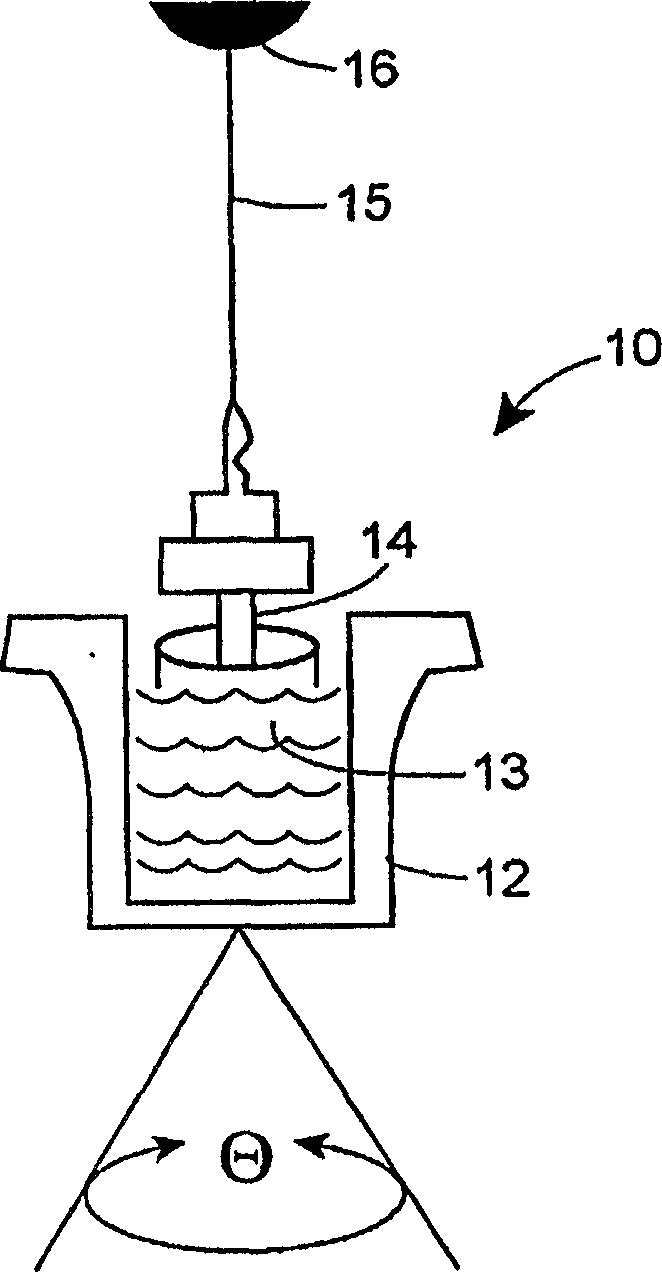
Protocol and apparatus for determining heparin-induced thrombocytopenia
A technology for thrombocytopenia and platelet activation, which is used in biochemical equipment and methods, microbial determination/inspection, material inspection products, etc., and can solve the problems of impossible detection of anticoagulant degree, high cost, and uncontrollable bleeding.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0041] Example 1: Determination of HiT II using platelet-rich plasma from normal donors mixed with citrated plasma from HiT II patients
[0042] Whole blood from a normal blood donor was drawn and transferred to a plastic centrifuge tube, which contained 5 μl˜15 μl of PPACK per 1 ml of blood. Normal blood donors should have a platelet count greater than 150,000 / μl and are not taking NSAIDS (non-steroidal anti-inflammatory drugs) or other platelet inhibitors. For a typical assay, 12 ml of blood is drawn and added to a tube containing about 180 μl of PPACK, which is capped and mixed by inversion several times, eg 3 times. The samples were centrifuged at 100 g for 20 minutes (800 RPM (revolutions per minute) for a standard IEC benchtop centrifuge) to separate platelet rich plasma (PRP) from other blood cells. This procedure will yield enough PRP for 3 to 4 assay mixtures, and excess PRP can be stored at -35°C to -70°C until assayed.
[0043] Prepare the PRP-patient plasma mixtu...
Embodiment 2
[0050] Example 2: Determination of HiT II using patient whole blood
[0051] A 3 ml sample of whole blood from a HiT II suspect patient was drawn into a plastic tube containing approximately 5 μl of 5 mg / ml PPACK per 1 ml of blood. The patient's platelet count should be greater than 50,000 / μl. Additionally, patients should not take GPIIb / IIIa inhibitor drugs such as Reopro(R), Integrilin(R), and Aggrestat, or other drugs that mask platelet activation by the HiT II antibody complex.
[0052] For a typical assay, 6 ml of patient blood is drawn into a plastic tube containing 30 μl of PPACK and mixed. This provides enough blood sample for assay and enough remaining blood sample for separation of plasma to be used for confirmatory testing with normal donor blood as described in Example 1 above, especially if the patient's platelet count is less than 50,000 / μl.
[0053] Prepare four sample containers for testing. Add 5 µl of saline to the first sample container. Add 5 μl of 1 U / ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com