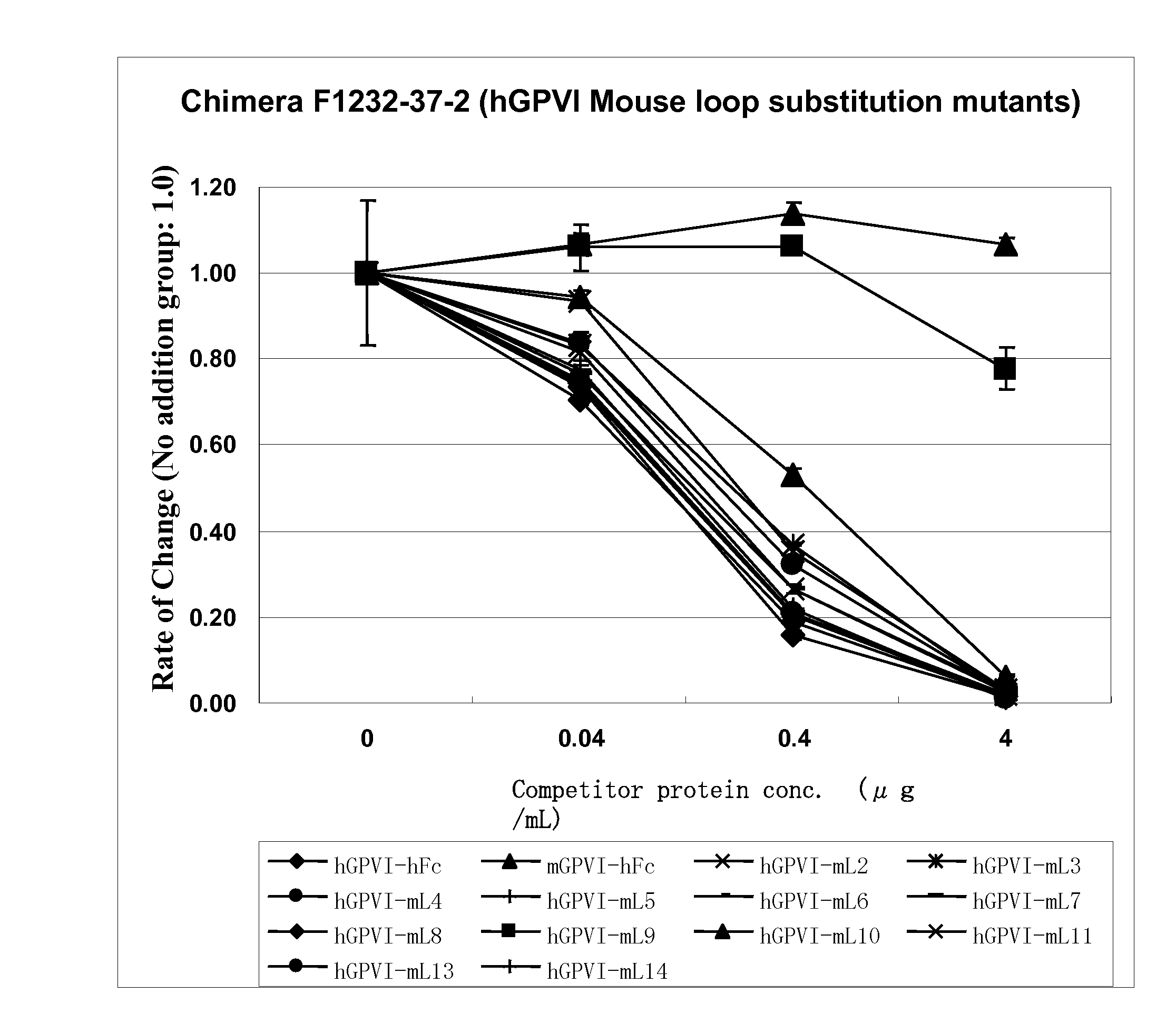
Anti-platelet membrane glycoprotein vi monoclonal antibody
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Preparation of GPVI Extracellular Region-Fc Fusion Protein
[0140]A. Preparation of Human GPVI Extracellular Region-Mouse Fc Fusion Protein (hGPVI-mFc)
(1) Construction of the Expression Plasmid for hGPVI-mFc Fusion Protein
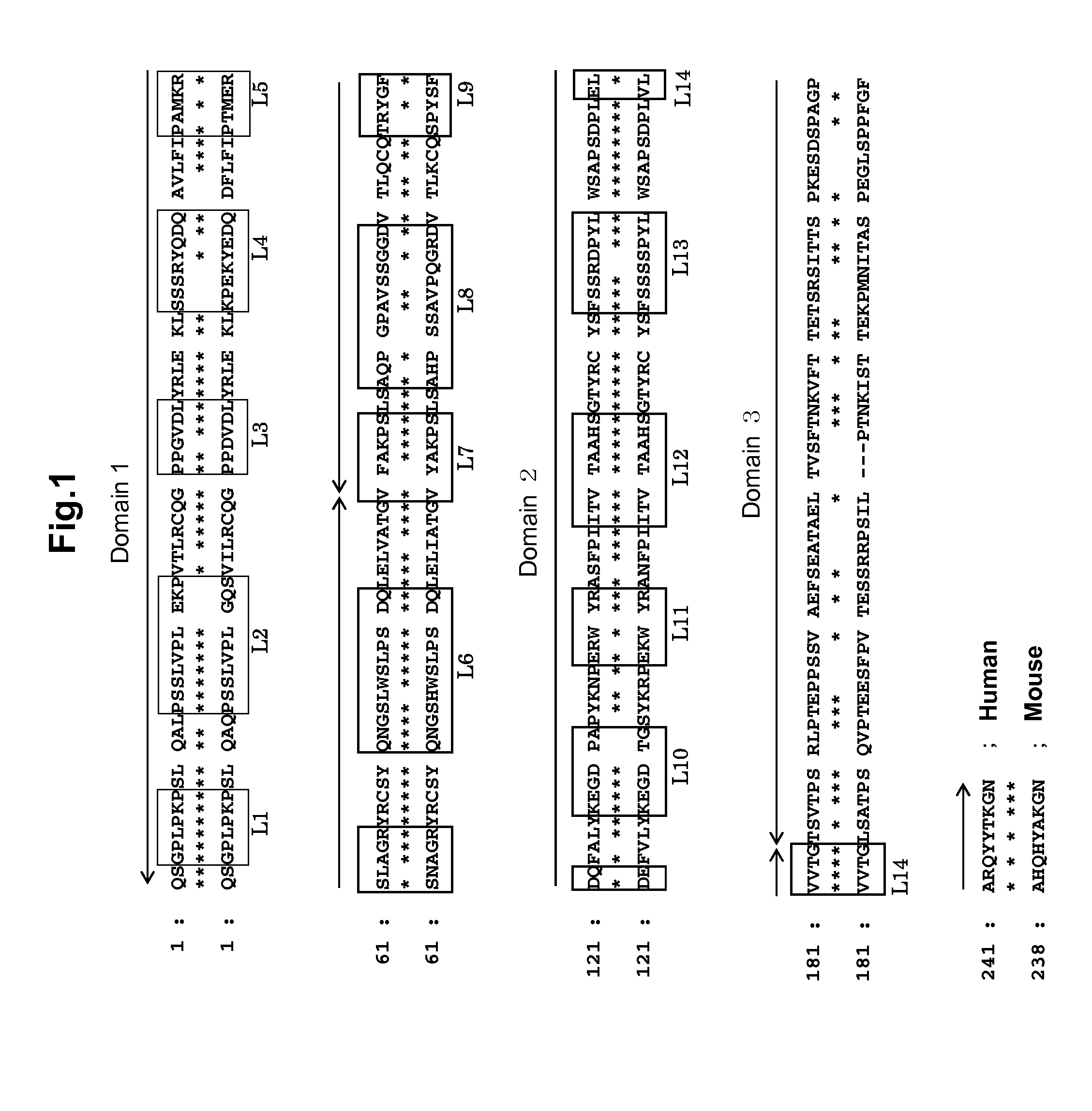
[0141]Using mouse genomic DNA as a template, gene regions encoding respective domains of mouse immunoglobulin (mIgG2a) heavy chain constant region were amplified. That is, with the following primer pair, PCR reaction was performed. As a result, CH1 domain, hinge region, CH2 domain and CH3 domain were amplified with mIgG2a-a (SEQ ID NO: 152) and mIgG2a-c (SEQ ID NO: 154), mIgG2a-b (SEQ ID NO: 153) and mIgG2a-e (SEQ ID NO: 156), mIgG2a-d (SEQ ID NO: 155) and mIgG2a-g (SEQ ID NO: 158), and mIgG2a-f (SEQ ID NO: 157) and mIgG2a-h (SEQ ID NO: 159), respectively. Then these four amplified products were mixed, and PCR reaction using primers mIgG2a-a and mIgG2a-h was performed to obtain an amplified product, wherein respective domains were ligated (DNA fragment encoding heavy...
example 2
Preparation of Anti-GPVI Antibody
A. Preparation of Rabbit Polyclonal Antibody
[0146]To prepare a polyclonal antibody to human GPVI, rabbit was immunized. That is, 20 μg of hGPVI-mFc prepared in EXAMPLE 1A was diluted with 500 μl of saline and mixed with 500 μl of Freund complete adjuvant (DIFCO). Then, the mixture was subcutaneously administered to the dorsal of female New Zealand white rabbit (KITAYAMA LABES) whose weight was 2.1-2.2 kg. Two weeks later, once again, 20 μg of hGPVI-mFc was diluted with 500 μl of saline and mixed with 500 μl of Freund incomplete adjuvant (DIFCO). Then, the mixture was subcutaneously administered to the dorsal. One week after the completion of administration, the blood was collected from the ear vein, and according to the conventional method, anti-serum was isolated to purify an antibody. That is, to the anti-serum ammonium sulfate was added to make the final concentrations of saturated solution 33%. After stirring at 4° C. for one hour, the precipitat...
example 3
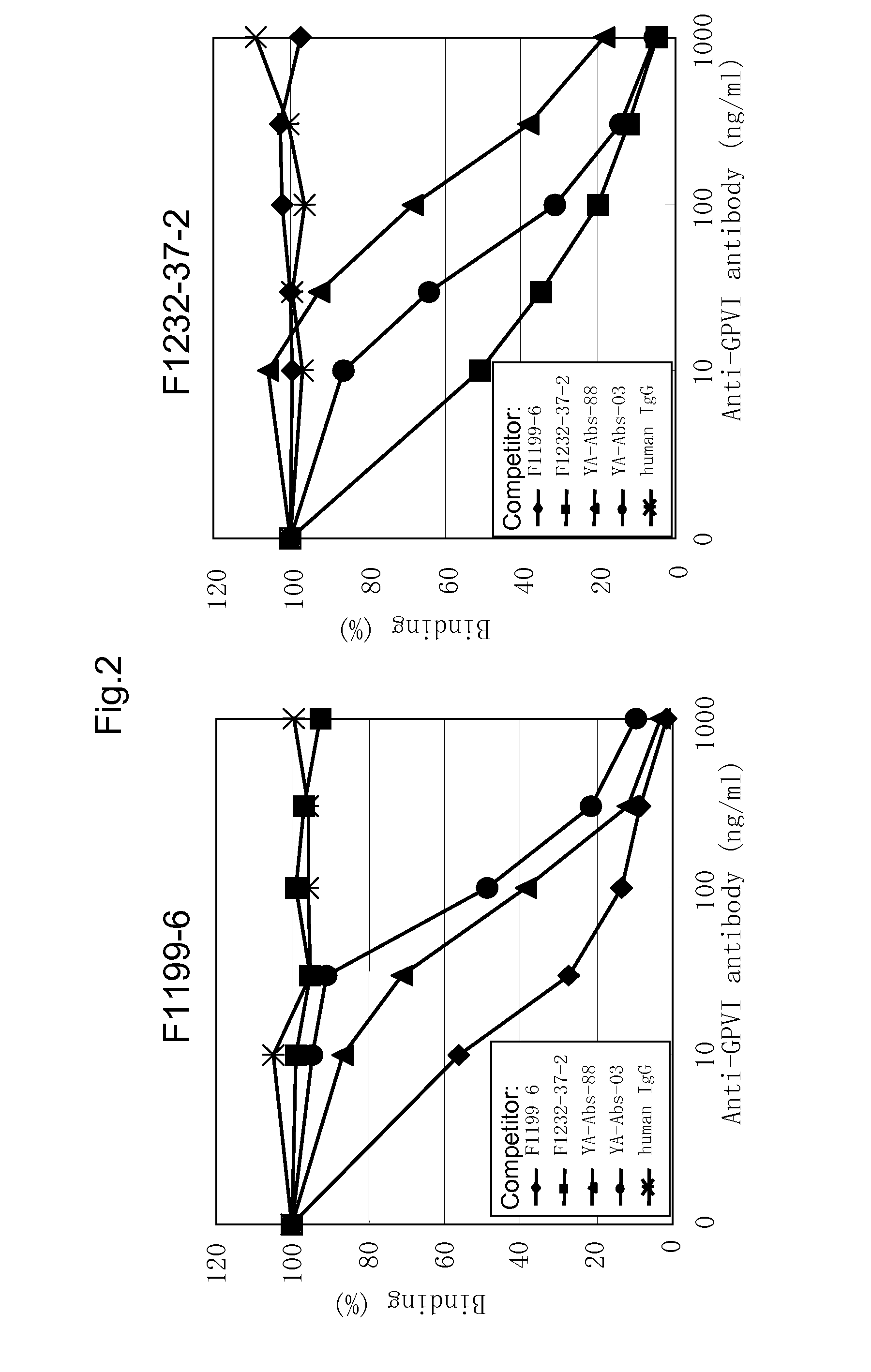
Group Classification of the Anti-GPVI Monoclonal Antibody
[0152]In order to classify the antibody obtained in EXAMPLE 2 by the binding property to GPVI, i.e. the difference of the binding region, using F1199, F1201, F1202, F1210 and F1211 antibodies, respectively, competitive assay was performed. Firstly, according to the method described by Nakane et al. (J. Histochem. Cytochem., 22, 1084, 1974), each peroxidase-labeled anti-GPVI monoclonal antibody was prepared.
[0153]Then, using the peroxidase-labeled antibody, competitive assay for respective purified antibodies. That is, hGPVI-hFc was diluted with D-PBS to 2 μg / mL, and the diluted solution was added to immunoplate (Maxisorp, NUNC) at 50 μL / well. After reaction at 37° C. for one hour, the well was washed five times with ion-exchanged water, and D-PBS containing 2% StabilGuard was added to each well for blocking. Then, 25 μL each of the above-mentioned labeled antibody and the purified antibody were added to the well and incubated ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Dissociation constant | aaaaa | aaaaa |
| Fraction | aaaaa | aaaaa |
| Fraction | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com