Quality control reference substance for antelope's horn tablets for common cold and application of quality control reference substance
A technology of Ganmao tablets and reference products, which is applied in the chromatographic conditions stipulated in the standard and conforms to all chromatographic columns. The field of quality control reference products of Lingyang Ganmao tablets achieves the effect of strong adaptability and great application potential.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] 1 Instruments and reagents
[0030] Instruments: Agilent 1200 high-performance liquid chromatography; Sartorius CP225D electronic analytical balance; BK-600C ultrasonic cleaner (Barker Ultrasonic Equipment Co., Ltd.).
[0031] Reagent: chlorogenic acid (110753-200414), vitexin rhamnoside (111687-200602), isorhamnetin-3-O-neohesperidin (111571-200402), forsythin (110821-200508 ), forsythiaside A (111810-201001, with a content of 94.3%), and arctiin (110819-201007, with a content of 94.6%) were all provided by the China Institute for Food and Drug Control. Acetonitrile was chromatographically pure.
[0032] 2 Methods and results
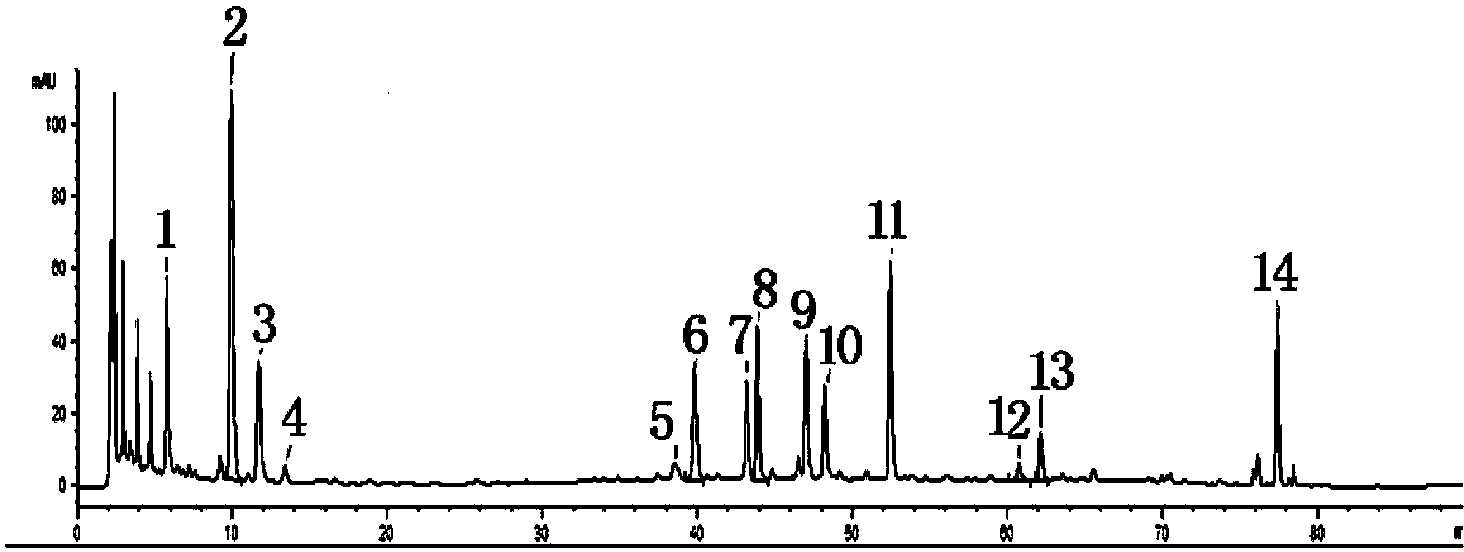
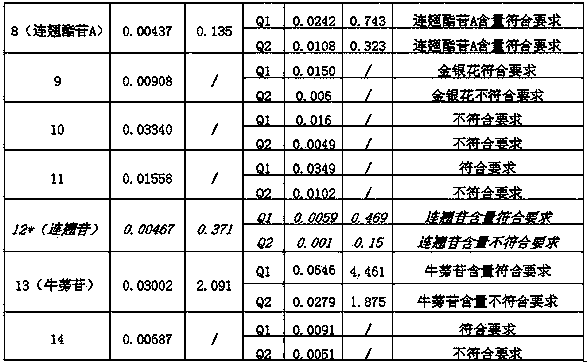
[0033] 2.1 Selection of reference products
[0034] 2.1.1 Preparation of mixed reference solution
[0035] Prepare a certain concentration of chlorogenic acid, liquiritin, vitexin rhamnoside, isorhamnetin-3-O-neohesperidin, forsythiaside A, forsythin and arctiin reference substance 50% methanol For the stock solution, accurately measure an ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com