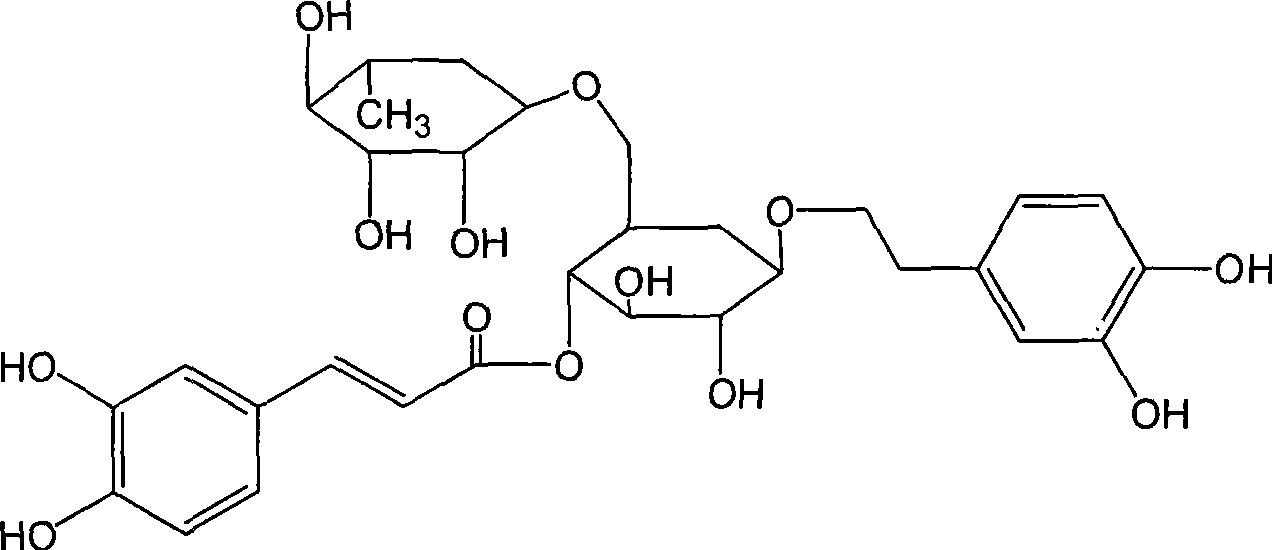
Use of high-purity forsythin in preparing bacteriostasis, antivirus and other medicine
An antiviral drug, the technology of forsythiaside, applied in antiviral agents, antibacterial drugs, drug combinations, etc., can solve the problems of antibacterial and antiviral, antipyretic and anti-inflammatory pharmacological activities of forsythiaside, etc. Achieve antibacterial and antiviral effects, enhance immunity, and inhibit proliferation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
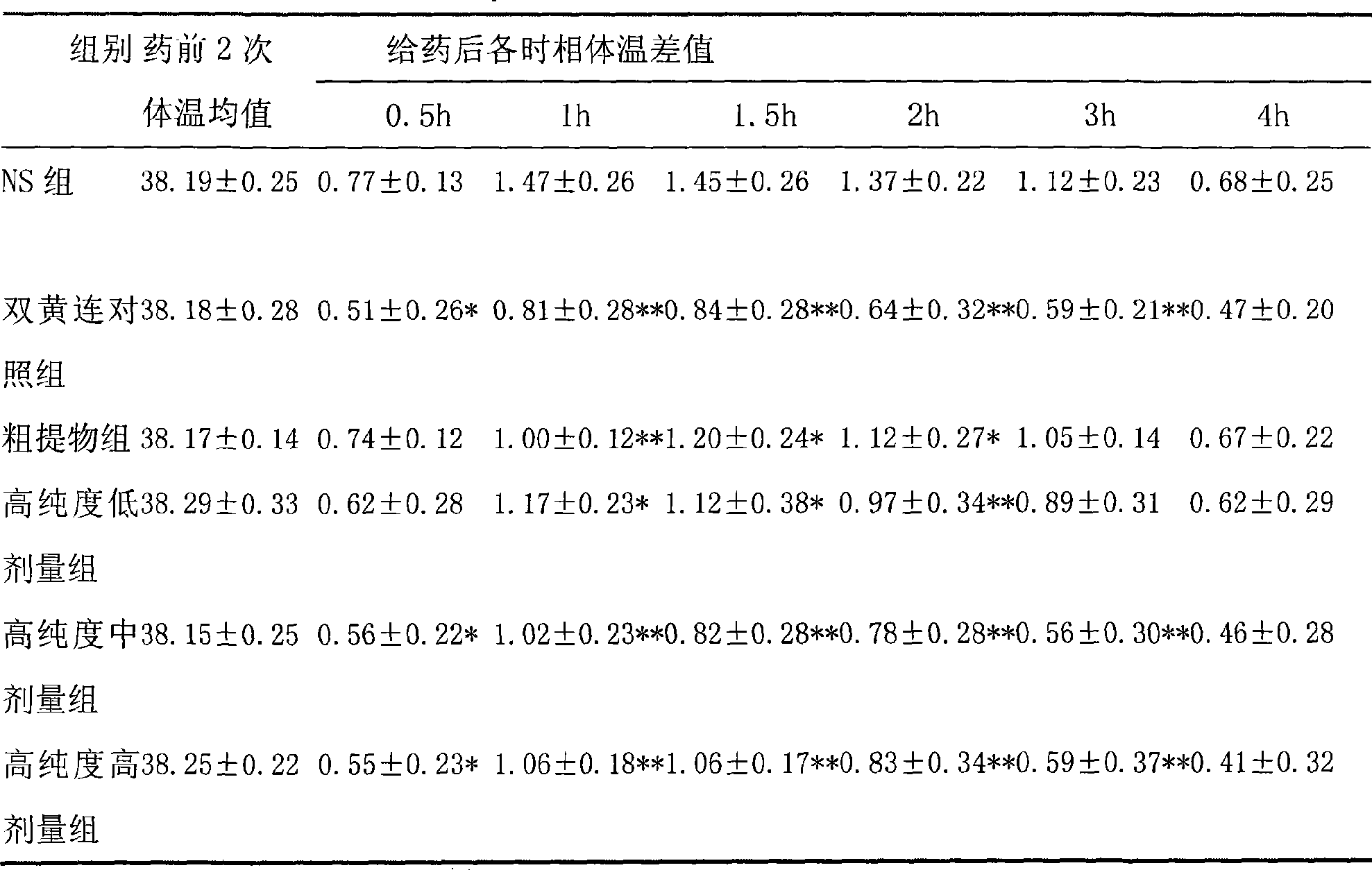
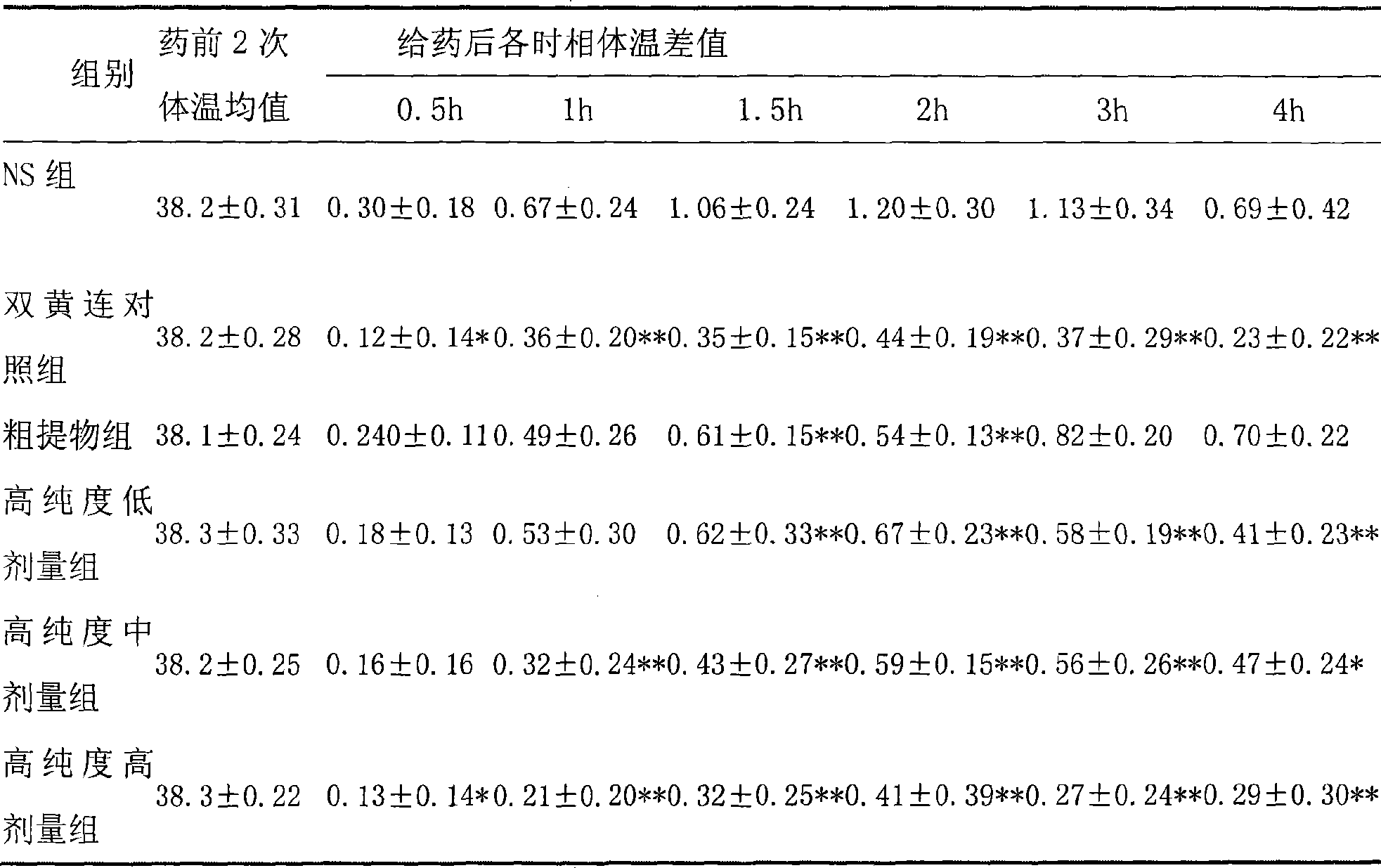
[0013] Embodiment 1 (antipyretic, anti-inflammatory)
[0014] 1.1 Purpose of the experiment
[0015] Observe the use of high-purity forsythiaside in antipyretic and anti-inflammatory aspects.
[0016] 1.2 Experimental materials
[0017] 1.2.1 Drugs
[0018] Test drug: high-purity forsythiaside: the active ingredient extract of forsythia; purity >90%; provided by Shanghai Yusen New Drug Development Co., Ltd. Physical and chemical properties: This product is yellow powder. Storage conditions: 4°C, protected from light. Batch number: 060425.
[0019] Three dose groups (hereinafter referred to as high-purity groups) were set up in the test. ① Rat administration preparation method: according to 10ml / (kg d) tail vein injection administration, the low, middle and high dose groups are respectively equivalent to 3, 6, 12 times of clinical daily dosage; ② Mice administration preparation method : Administered at 10ml / (kg·d), the low, middle and high dose groups are respectively eq...
Embodiment 2
[0063] Embodiment 2 (enhancing immunity test)
[0064] 2.1 Purpose of the experiment
[0065] Observe the use of high-purity forsythiaside in enhancing immunity.
[0066] 2.2 Experimental materials
[0067] With 1.2 experimental material in embodiment 1
[0068] 2.3 Data processing
[0069] Same as 1.3.
[0070] 2.4 Methods and Results
[0071] 2.4.1 Effect of high-purity forsythiaside on the carbon clearance ability of mice
[0072] The grouping was the same as in 1.4.1. Continuous tail vein injection of 10ml / kg was administered for 1 week. The mice in each group were injected with 0.1ml of 20% India ink through the tail vein respectively, and 20ul of blood was collected at 1 minute and 5 minutes after the injection and placed in 2ml0. Mix in 1% Na2CO3 solution, measure the OD value at 680nm, and calculate the K value according to the following formula.
[0073] Formula: K=(logOD1-logOD5) / (t5-t1)
[0074] The results showed that there was a significant difference betw...
Embodiment 3
[0086] The results of this experiment show that high-purity forsythiaside can better increase the charcoal clearance ability of mice, inhibit the transformation of PHA on mouse lymphocytes, and have the effect of enhancing immune resistance; while the crude extract of forsythiaside does not have this effect. effect. Embodiment 3 (in vitro antiviral test)
[0087] 3.1 Virus multiplication:
[0088] Inoculate influenza type A on MDCK cells, add maintenance solution and culture at 37°C, 5% CO2, more than 90% of the lesions appear after 96 hours, freeze and thaw 3 times, blow and centrifuge, quantitatively aliquot, and store in -80°C refrigerator for later use .
[0089] 3.2 Determination of virus virulence:
[0090] Make a 10-fold serial dilution of the spare influenza A virus with the maintenance solution, repeat 3 wells vertically, inoculate horizontally on the monolayer cells in a 96-well plate, culture at 37°C, 5% CO2, observe the lesions every day, 48 After -96h, suck an...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com