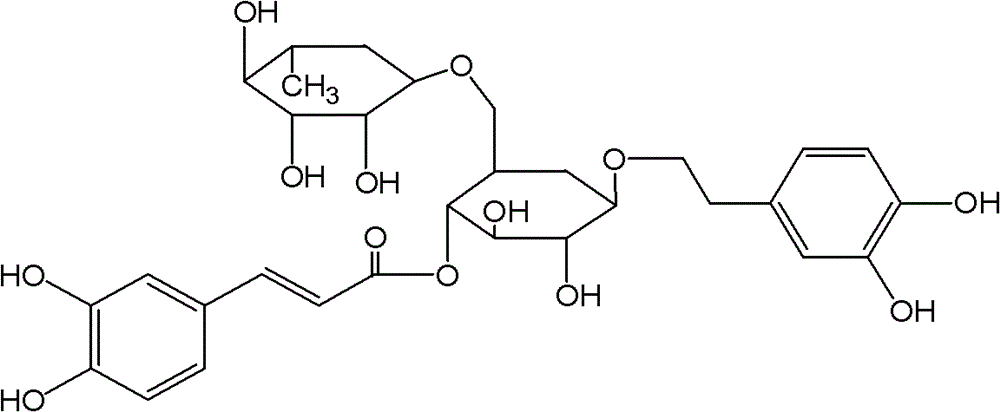
Application of Forsythoside A in preparation of anti-influenza A H1N1 virus medicine and preparation thereof
A technology of forsythiaside and medicine, which is applied in the preparation of anti-influenza A (H1N1) virus drugs and its preparation field, and can solve problems that do not involve the application of forsythiaside in anti-influenza A (H1N1) virus drugs, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
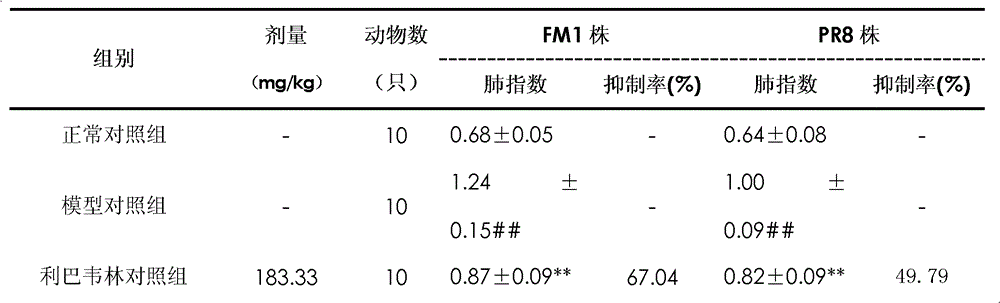
[0024] Example 1 In vivo pharmacodynamic test of forsythiaside in the treatment of respiratory virus infection
[0025] 1. Test purpose
[0026] To investigate the effectiveness of forsythoside in the treatment of respiratory virus infections, and to provide a basis for the research, development and clinical application of forsythoside.
[0027] 2. Test site
[0028] ABSL-2 Biosafety Laboratory, Institute of Chinese Materia Medica, China Academy of Chinese Medical Sciences.
[0029] 3. Test materials
[0030] 3.1 Test drug
[0031] Forsythiaside, provided by Shanghai Yusen New Drug Development Co., Ltd., batch number: 090801, date of receipt: 2010.07.12, expiration date: tentatively 2 years; content: 94%, properties: light yellow powder, solubility: easily soluble in water , Storage conditions: sealed refrigerator at 4℃.
[0032] 3.2 Positive control drug
[0033] 3.2.1 Ribavirin injection: product of Tianjin Pharmaceutical Jiaozuo Co., Ltd., production date: 2009.11.07, batch number: 0911...
Embodiment 2
[0072] Example 2 In vitro anti-respiratory virus pharmacodynamic test of forsythiaside
[0073] 1. Test purpose
[0074] Observe the effect of forsythiaside against respiratory viruses in vitro.
[0075] 2. Test site
[0076] ABSL-2 Biosafety Laboratory, Institute of Chinese Materia Medica, China Academy of Chinese Medical Sciences.
[0077] 3. Test materials
[0078] 3.1 Cells: Dog kidney cells (MDCK), human laryngeal carcinoma epithelial cells (Hep-2) and human cervical cancer cells (Hela) were purchased from the Cell Center of the Institute of Basic Medicine, Peking Union Medical College, and were routinely subcultured in our room.
[0079] 3.2 Viruses: A H1N1 influenza virus (PR8 strain, brisbane / 59 / 2007 strain), B influenza virus Jiangxi Xiushui strain, purchased from the influenza room of the Viral Disease Prevention and Control Institute of the Chinese Center for Disease Control and Prevention, and conventional chicken embryo passage in this room , TCID50 is 10 -4.5 , 10 -4 , 10 -...
Embodiment 3
[0124] Example 3 Preparation of freeze-dried powder injection
[0125] prescription:
[0126]
[0127] Preparation process: Take 50g of forsythiaside, 50g of glucose, and 150g of sorbitol, add 1000ml of water for injection, stir to dissolve, adjust pH to 5.0 with citric acid-disodium hydrogen phosphate buffer salt, and remove with 0.22μm microporous membrane Bacteria filtration, then use 8000 Dalton ultrafiltration membrane to remove pyrogen; after the intermediates are qualified, fill up the volume with water for injection, divide them into 1000 pieces, put them in a freezer, and freeze-dry according to the following freeze-drying curve.
[0128] Temperature(℃)
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com