A method for detecting the pathogenicity of influenza A h1n1 virus based on pyrosequencing
A pyrosequencing, influenza virus technology, applied in the direction of determination/inspection of microorganisms, biochemical equipment and methods, etc., can solve the problems of laborious, time-consuming detection of virus gene mutations, etc., to achieve simple operation, prevent the further expansion of the epidemic, and quickly The effect of the experimental protocol
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
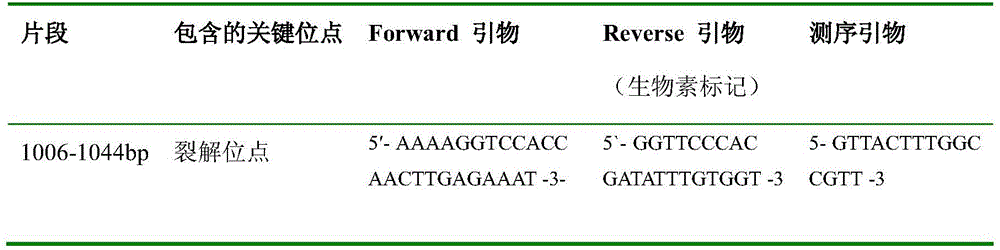
[0040] The content of the present invention will be further described in detail below by way of examples.
[0041] 1. RNA extraction
[0042] Use freshly propagated virus-infected MDCK passage cells as templates for RNA extraction, and use the HighPureViralRNAKit produced by Roche to extract viral RNA. The specific operation is carried out according to the kit instructions. The extracted RNA is immediately used for RT-PCR amplification or stored at -80°C. .
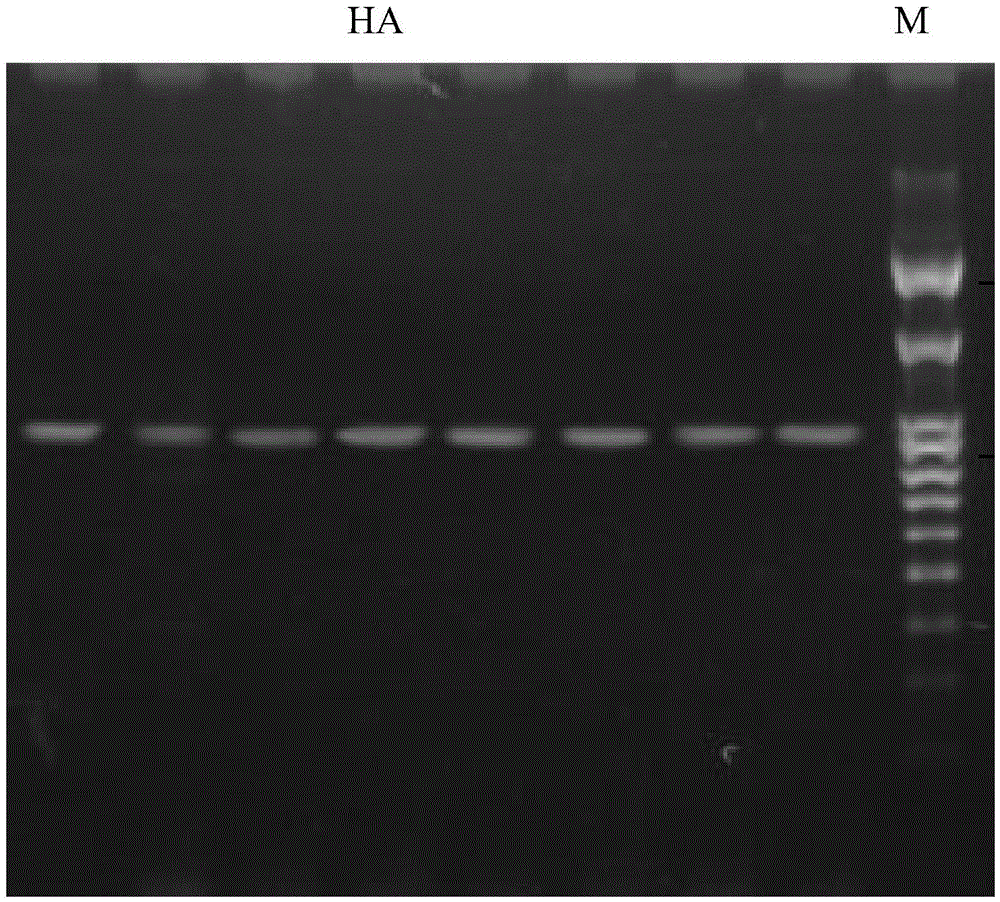
[0043] 2. RT-PCR reaction to amplify the HA1 gene fragment
[0044] The first round of amplification was carried out with the PCR primers having the sequence indicated by H1F and the PCR primers having the sequence indicated by H1R, and the extracted viral RNA was used as a template. In the 50μL reaction system, the optimal concentration of the first primer is 50nmol / L. The RT-PCR reaction conditions were: reverse transcription at 50°C for 30min; pre-denaturation at 94°C for 2min; 45 cycles of 94°C for 15s, 55°C for 30...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com