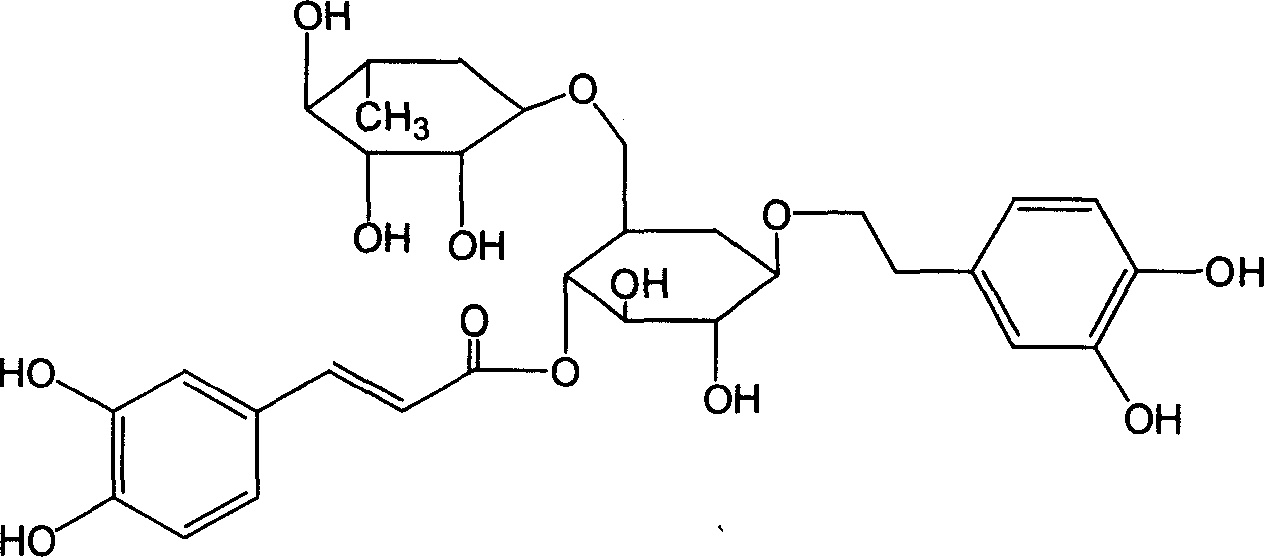
Method for purifying forsythiaside A from forsythia extractive
A technology of forsythia extract and forsythiaside, applied in the field of medicine, can solve the problems of reducing the extraction efficiency, limiting the extraction process of forsythiaside A, etc., and achieves the effect of improving efficiency and saving and utilizing raw materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0016] Get the Forsythia extract, mix the sample and put it on a 100-mesh silica gel column, use ethyl acetate-acetone-water (8:2:0.5) as the mobile phase to elute, collect 100ml of each fraction, combine 13-21 fractions, Concentrate to dryness under reduced pressure, and the residue is subjected to thin-layer silica gel column chromatography, eluting with ethyl acetate-acetone-water (8:2:0.5) as the mobile phase, collecting 10ml of each fraction, and combining 5-15 fractions, Concentrated to dryness under reduced pressure, the residue was purified by RP-18 silica gel column, eluted with methanol-water (3:7), 10ml was collected for each fraction, 47-49 fractions were combined, concentrated to dryness under reduced pressure, and dried in vacuo, that is Forsythiaside A was obtained.
Embodiment 2
[0018] Get the Forsythia extract, put it on a 200-mesh silica gel column after mixing the sample, and use ethyl acetate-methanol-water (7:2:1) as the mobile phase to elute, collect 100ml of each fraction, combine 12-21 fractions, Concentrate under reduced pressure to dryness, and the residue is subjected to thin-layer silica gel column chromatography, eluting with ethyl acetate-methanol-water (7:2:1) as the mobile phase, collecting 10 ml of each fraction, and combining 6-15 fractions, Concentrated to dryness under reduced pressure, the residue was purified by RP-18 silica gel column, eluted with methanol-water (2:8), 10ml of each fraction was collected, 47-49 fractions were combined, concentrated to dryness under reduced pressure, and dried in vacuo, that is Forsythiaside A was obtained.
Embodiment 3
[0020] Take the Forsythia extract, mix the sample and put it on a 200-mesh silica gel column, elute with chloroform-methanol-water (5:2.5:2.5) as the mobile phase, collect 100ml of each fraction, combine 12-21 fractions, and depressurize Concentrated to dryness, the residue was subjected to thin-layer silica gel column chromatography, eluted with chloroform-methanol-water (5:2.5:2.5) as the mobile phase, and 10ml of each fraction was collected, 6-15 fractions were combined, and concentrated under reduced pressure to Dry, the residue is purified by RP-18 silica gel column, eluting with methanol-water (2:8), collecting 10ml of each fraction, combining 47-49 fractions, concentrating under reduced pressure to dryness, and drying in vacuo to obtain Forsythia Glycoside A.
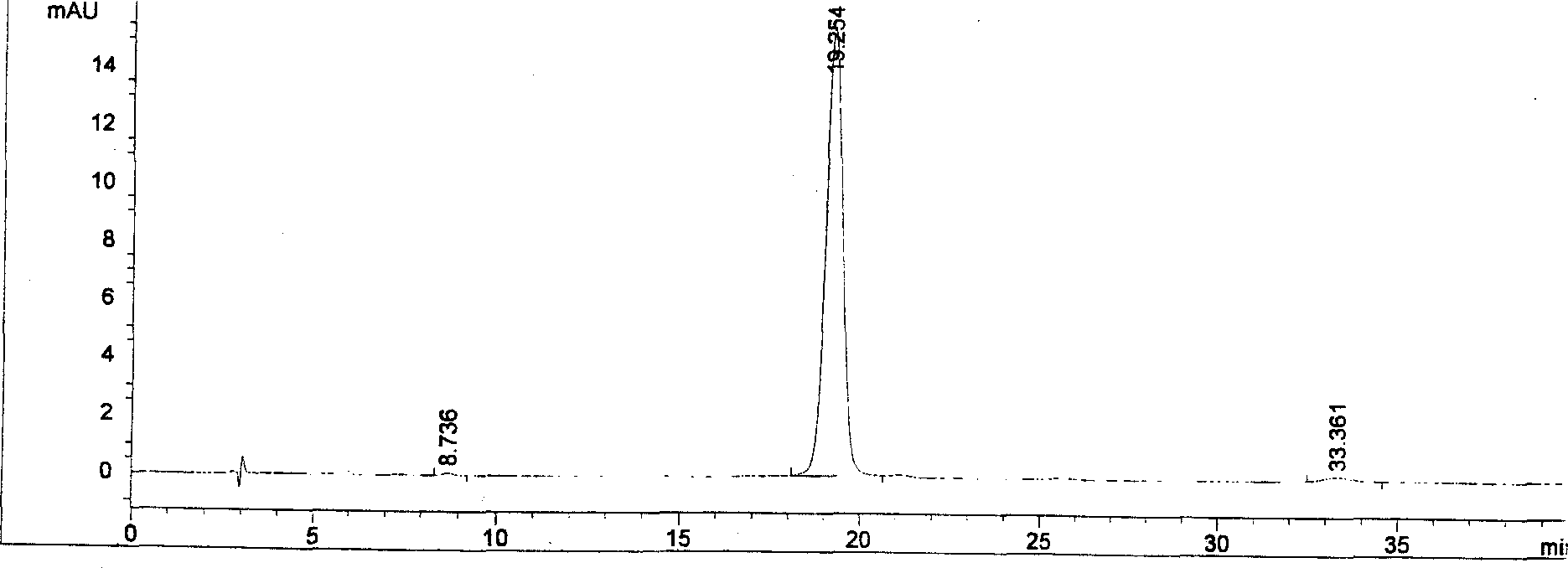
[0021] Identification of forsythiaside A
[0022] Accurately weigh an appropriate amount of forsythiaside A prepared according to the method in the example, add methanol to make a solution containing 0.62 mg of ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com