Method for determining content of chemical components in chrysanthemum
A technology of chemical composition and content, applied in the field of determination of chemical composition content in chrysanthemum, can solve the problems of imperfect quality control system and large quality difference of chrysanthemum medicinal materials, and achieve the effect of high sensitivity, strong specificity and fast analysis speed
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0041] 1. Determine the chromatographic conditions
[0042] Chromatographic column: ACQUITY UPLC BEH C18 column (2.1×100mm, 1.7μm); mobile phase: phase A is 0.1% formic acid water, phase B is acetonitrile; gradient elution, elution program: 0-2min, 6%-25 %B; 2-4min, 25%-26%B; 4-7min, 26%-75%B, 7-8min, 75%-95%B. Flow rate: 0.3mL / min; column temperature: 20°C; injection volume: 5μL.
[0043] 2. Determination of mass spectrometry conditions
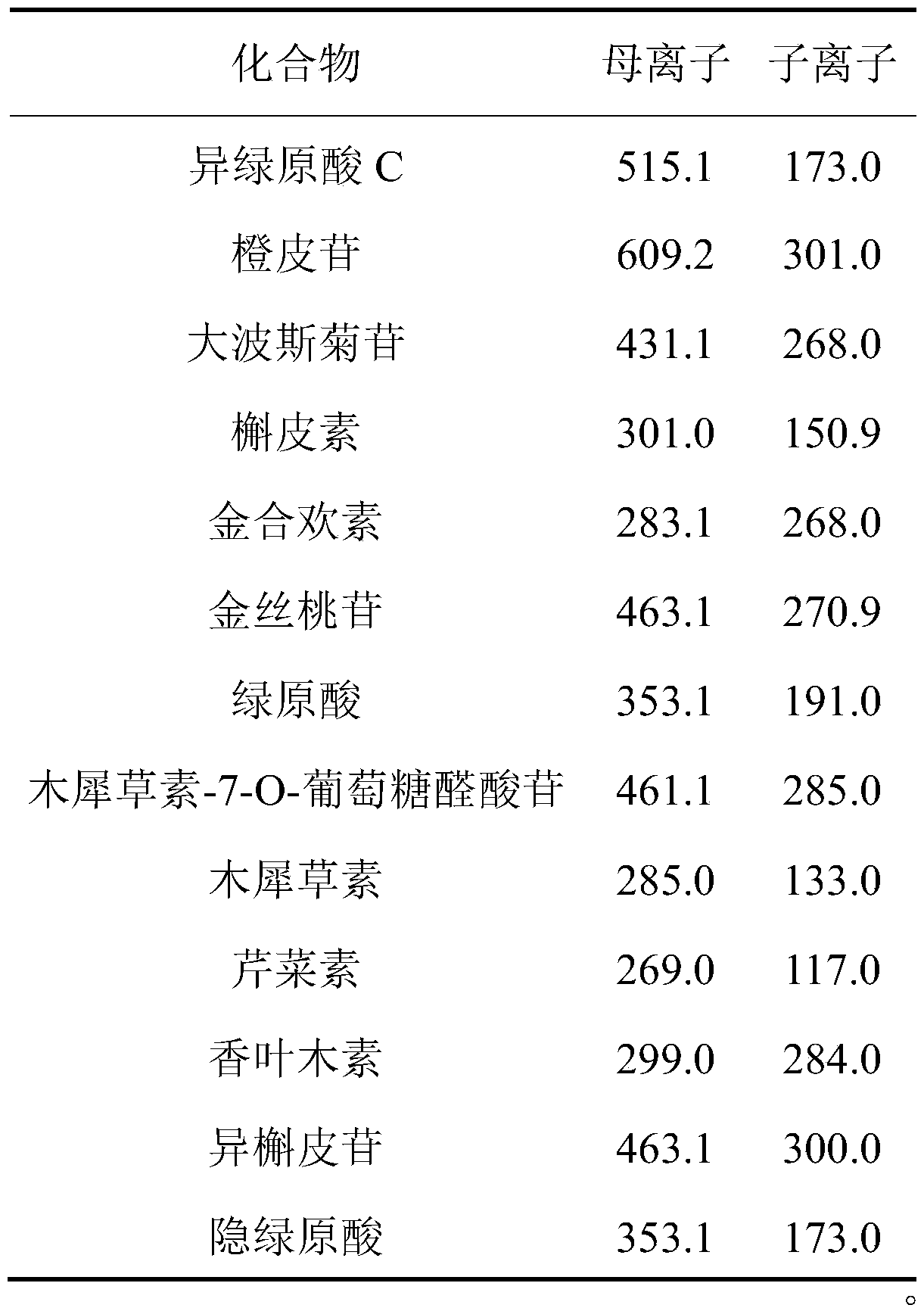
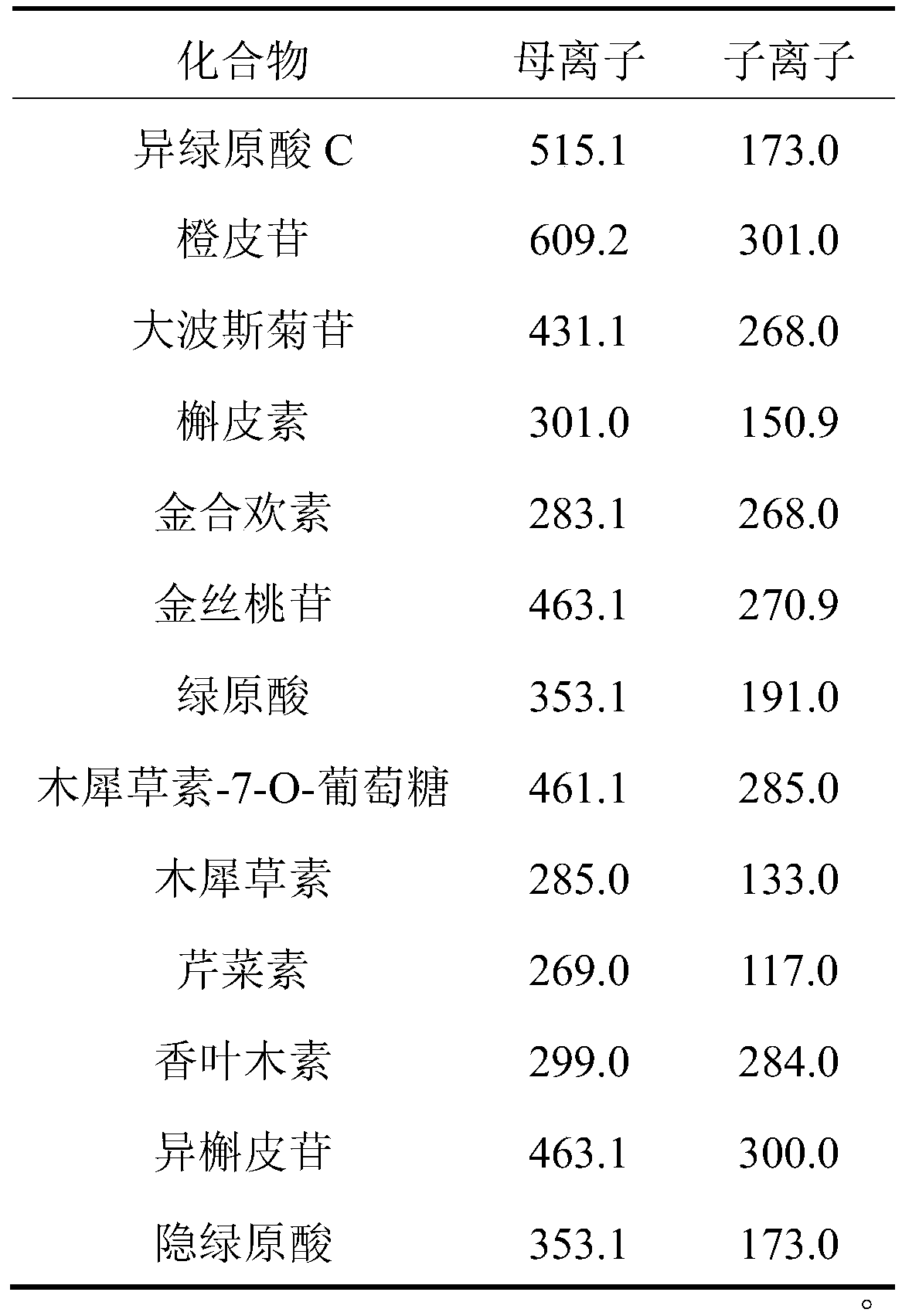
[0044] Electrospray ion source (ESI) is used, with multiple reaction ion monitoring (MRM) as the detection mode, negative ion full scan mode; capillary voltage: 4000V; capillary temperature is set to 300°C, dry gas flow rate is 7L / min; atomizer pressure is 35psi . The ion pairs and mass spectrometry parameters of the 13 analytes are shown in Table 1.
[0045] Table 1 Ion pairs and mass spectrometry parameters information of 13 compounds in chrysanthemum
[0046]
[0047] 3. Preparation of mixed reference substance stock solution
[...
Embodiment 2
[0054] Embodiment 2 establishes standard curve
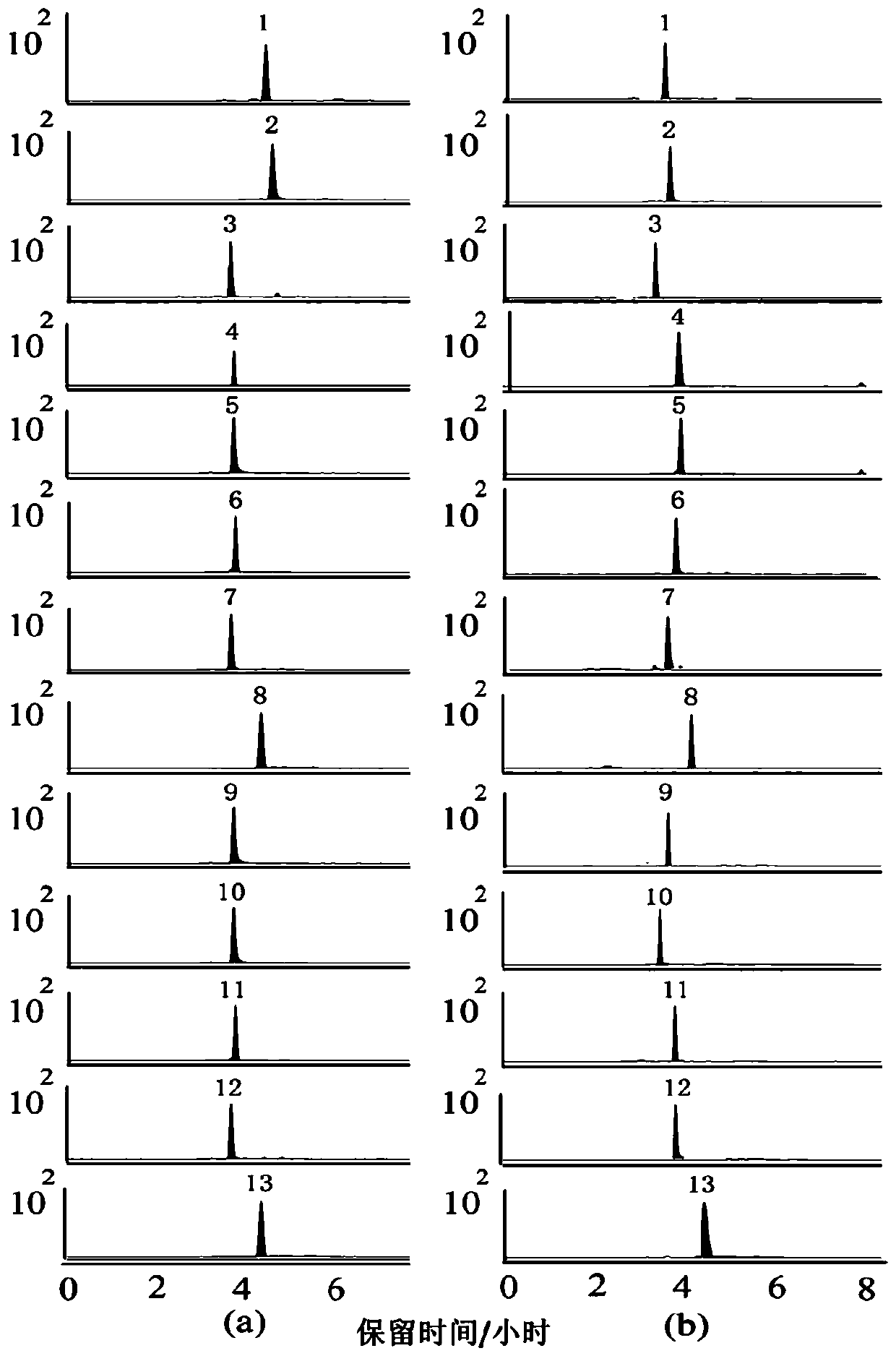
[0055] Accurately measure an appropriate amount of the mixed reference substance stock solution prepared in Example 1, use methanol as a solvent, and carry out gradient dilution of the mixed reference substance stock solution with 2, 2, 2, 2.5, 2, 2.5, 2 times successively to obtain 8 For the mixed reference substance solutions of four concentrations, 8 concentrations of mixed reference substance solutions were taken respectively, and 5 μL of sample injection was analyzed to obtain the multiple reaction ion monitoring spectra of each reference substance at different concentrations, wherein, the mixed control substance after the second dilution The multiple reaction ion monitoring chromatogram of the sample solution is as follows figure 1 As shown in figure a. With the concentration of the analyte (X) as the abscissa and the peak area of the analyte (Y) as the ordinate, the weighted least square method is used for regression c...
Embodiment 3
[0058] Embodiment 3 precision test
[0059] 1. Intra-day precision: prepare the mixed reference substance stock solution according to the method of Example 1, inject samples continuously for 6 times according to the chromatographic conditions and mass spectrometry conditions of Example 1, and obtain the peak area of each compound, see Table 4, record 13 kinds of chemical compounds The peak area of the components was used to calculate the RSD value, and the results showed that the intraday precision of this method was good.
[0060] Table 4 Intraday precision experiment data table of 13 components of chrysanthemum (n=6)
[0061]
[0062]
[0063] 2. Day-to-day precision: prepare the mixed reference substance stock solution according to the method in Example 1, repeat the sample injection 2 times according to the chromatographic conditions and mass spectrometry conditions in Example 1, and record the peak areas of 13 chemical components for three consecutive days , to...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com