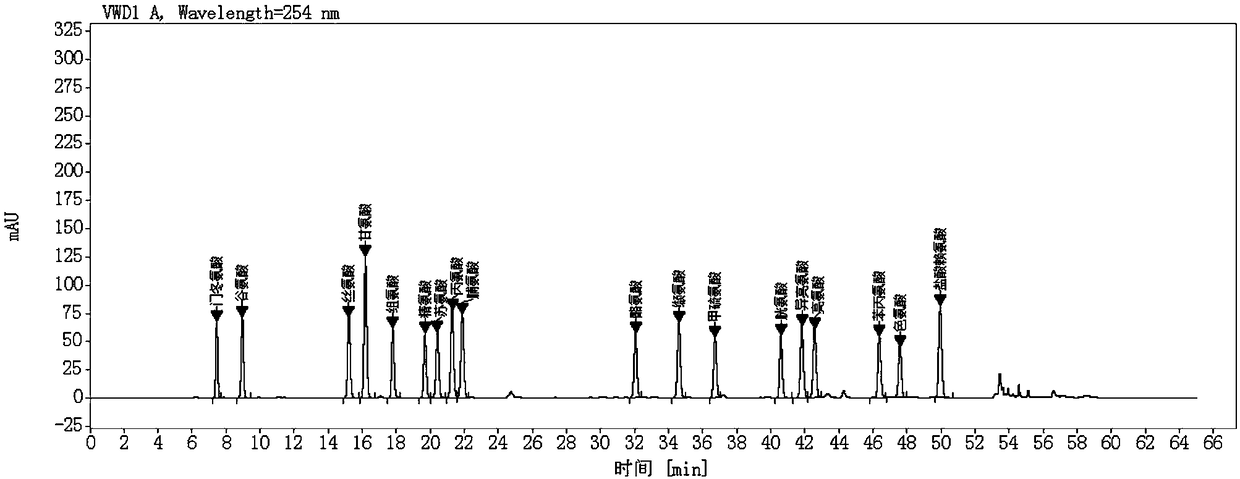
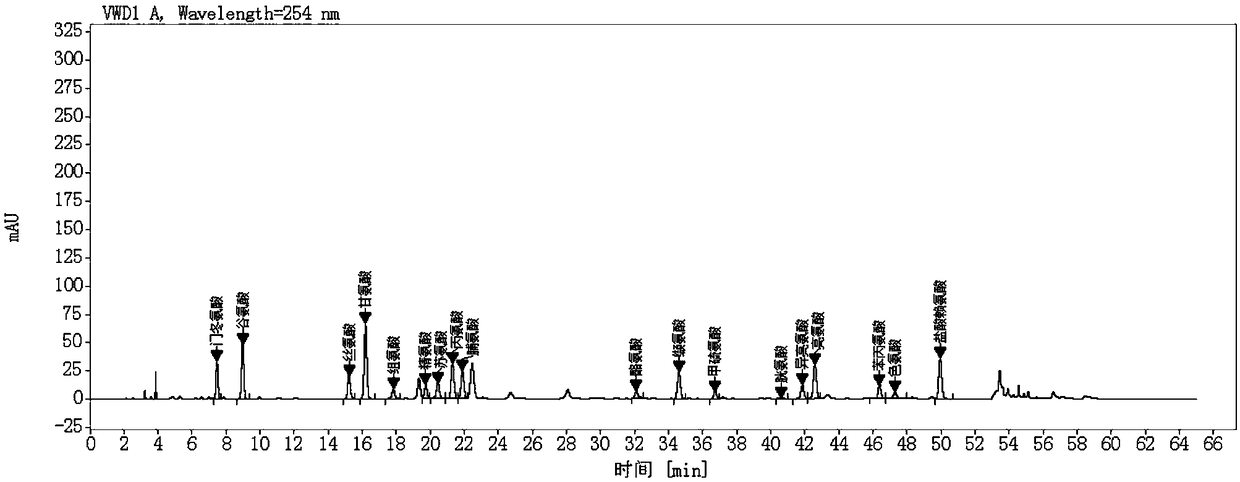
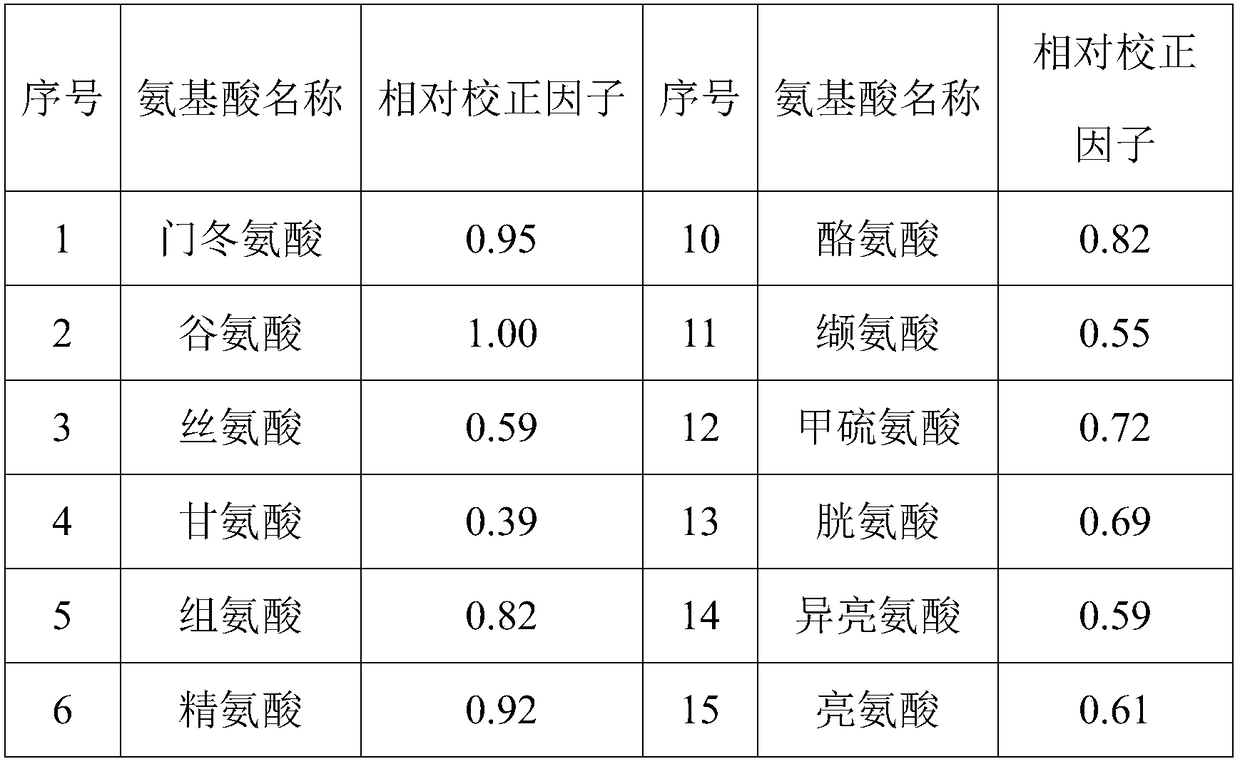
Method for quantificationally detecting total amino acids of transfer factor capsule
A quantitative detection method, the technology of total amino acids, applied in the field of quantitative detection of total amino acids of transfer factor capsules, can solve the problems of deviation of detection results, high detection cost, cumbersome operation, etc., to improve detection accuracy, realize supervision and control, and reduce overlap The effect of proportion
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0062] In step 1), the preparation method of transfer factor capsule total amino acid content assay need testing solution, specifically comprises the following steps:
[0063] a. Accurately weigh about 1.0 g of the contents of the transfer factor capsule, put it in a 20ml stoppered test tube, add 0.1mol / L hydrochloric acid solution, shake on an oscillator to dissolve the transfer factor, shake well, and let stand; take the supernatant for use Filter through a 0.45 μm microporous membrane, accurately measure 2.0 mL of the subsequent filtrate, put it in a digestion tube, add 6 mL of concentrated hydrochloric acid, seal it with nitrogen, place it at 110 ° C for 24 hours, and let it cool to prepare the transfer factor acid hydrolyzate.
[0064] b. Unseal the transfer factor acid hydrolyzate, put it in an evaporating dish, and evaporate to dryness in a water bath; add 10 mL of 0.1 mol / L hydrochloric acid solution to dissolve the residue, and use it as the test solution for the deter...
Embodiment 1
[0086] The screening of embodiment 1 transfer factor capsule hydrolysis conditions
[0087] The hydrolysis conditions of transfer factor capsules were screened by 3-factor 3-level orthogonal test. The 3 factors investigated for hydrolysis conditions were hydrolyzed sample concentration, hydrolyzed time, and hydrolyzed acid volume; the 3-factor 3 The horizontal orthogonal test table is shown in Table 1, and the analysis of the orthogonal test results is shown in Table 2:
[0088] Table 1 Orthogonal test table of transfer factor capsule hydrolysis condition screening
[0089] Level
Hydrolysis sample concentration A
Hydrolysis time B
Hydrolyzed acid volume C
1
2.0mg / ml
22h
3mL
2
2.4mg / ml
24h
6mL
3
2.8mg / ml
26h
9mL
[0090] Table 2 Analysis table of results of orthogonal test of transfer factor capsule hydrolysis condition screening
[0091]
[0092]
[0093] It can be seen from Table 2 that the o...
Embodiment 2
[0094] Example 2 The screening of chromatographic conditions for the determination of total amino acid content in transfer factor capsules
[0095] Three factors and three levels of orthogonal tests were used to screen the total amino acid content of transfer factor capsules and determine the chromatographic conditions. The three factors investigated in the chromatographic conditions were the composition ratio of mobile phase B, the column temperature of the chromatographic column, and the flow rate of the mobile phase; See Table 3 for the 3-factor 3-level orthogonal test table of the optimal level value design, and see Table 4 for the analysis of the results of the orthogonal test.
[0096] Table 3 Orthogonal test table for determination of total amino acid content in transfer factor capsules and screening of chromatographic conditions
[0097] Level
Moving Phase B Composition Ratio A
Mobile phase flow rate C
1
98:2
25℃
...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
| length | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com