Method for quantitatively detecting polypeptide in transfer factor capsule
A quantitative detection method and transfer factor technology, applied in the direction of measuring devices, instruments, scientific instruments, etc., can solve the problems of interference detection results, poor reproducibility, inaccurate detection results, etc., to achieve reduced overlap ratio, good peak shape, and improved The effect of detection accuracy
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0050] The preparation method of the test solution for the determination of the total amino acid content of transfer factor capsules of the present invention comprises the following steps:
[0051] a. Accurately weigh about 1.0 g of the contents of the transfer factor capsule, put it in a high-throughput microwave digestion tube, add 6-10 mL of 6mol / L hydrochloric acid solution precisely, weigh it, and put it in a microwave digestion apparatus for hydrolysis according to the procedure in the table below:
[0052] Table 1 Microwave digestion program for determination of total amino acids in transfer factor capsules
[0053] step
climb (min)
keep (min)
temperature(℃)
power (w)
1
5
5
100
1200
2
5
5
150
1200
3
5
15
180
1200
[0054]b. After the digestion process of the contents of the above transfer factor capsules is completed, let cool to room temperature, make up the lost weight with 6mol / L hydr...
Embodiment 1
[0077] Example 1 Screening of Transfer Factor Capsule Microwave Digestion Conditions
[0078] The microwave digestion conditions of transfer factor capsules were screened by 3-factor 3-level orthogonal test. The 3 factors of microwave digestion conditions were digestion sample volume, digestion acid volume and digestion temperature; the optimal level of 3 factors was determined by single factor test. The values are respectively 1.0 g of digested sample volume, 8.0 mL of digested acid volume, and 180°C of digested temperature. See Table 4 for the 3-factor 3-level orthogonal test table designed based on the optimal level values of the above 3 factors, and see Table 5 for the analysis of the orthogonal test results.
[0079] Table 3 Orthogonal test table of transfer factor capsule microwave digestion condition screening
[0080]
[0081]
[0082] Table 4 Analysis table of orthogonal test results for transfer factor capsule microwave digestion conditions screening
[0...
Embodiment 2
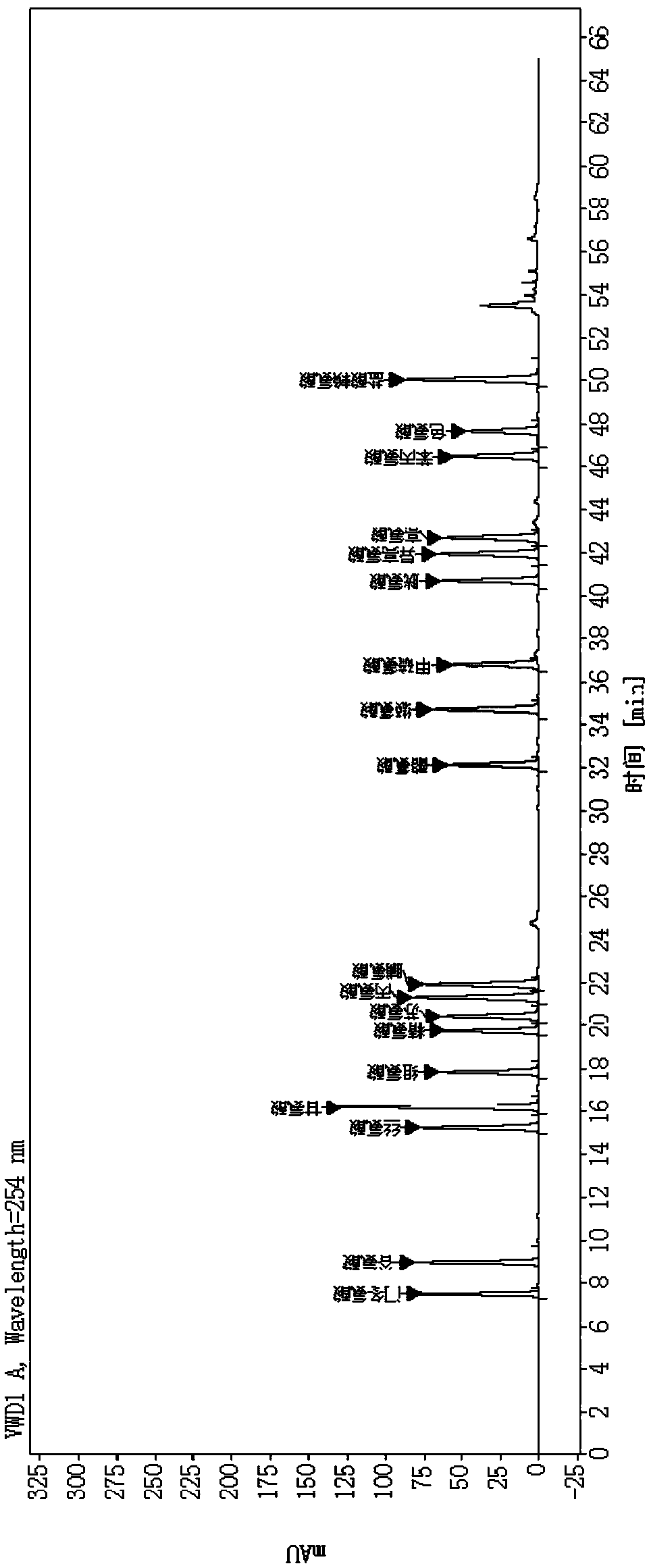
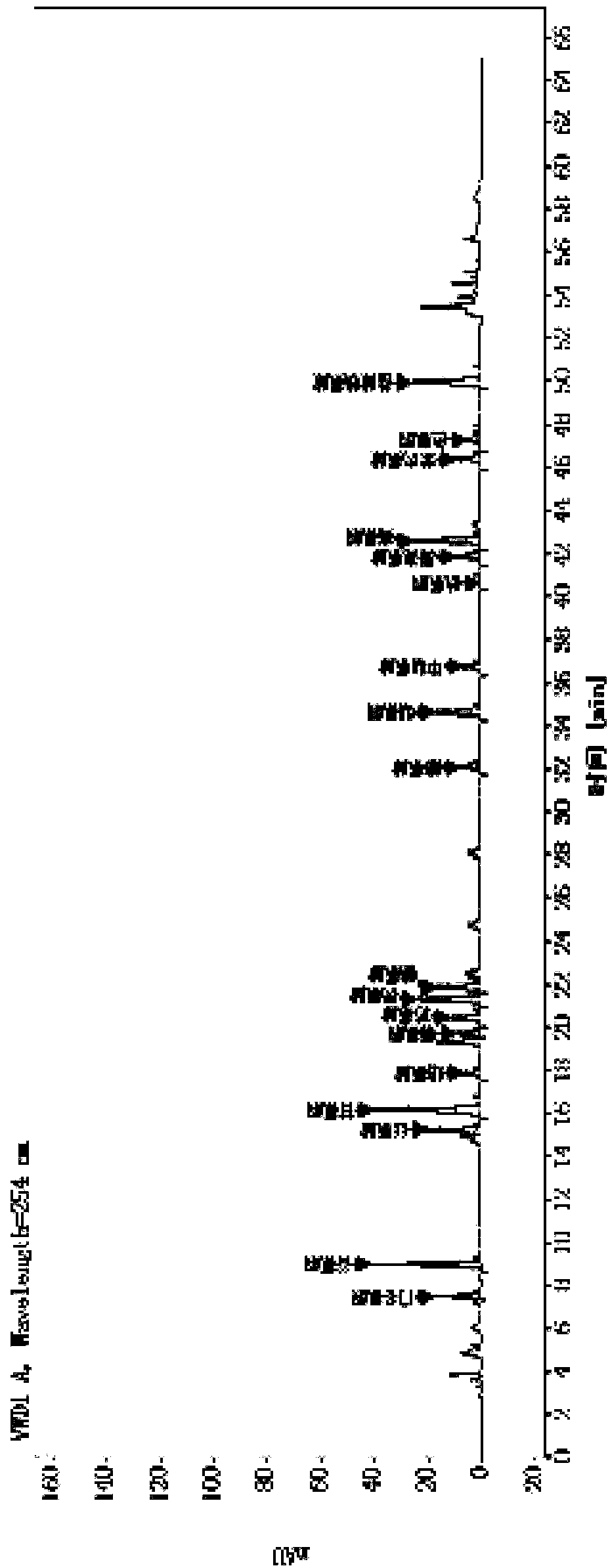
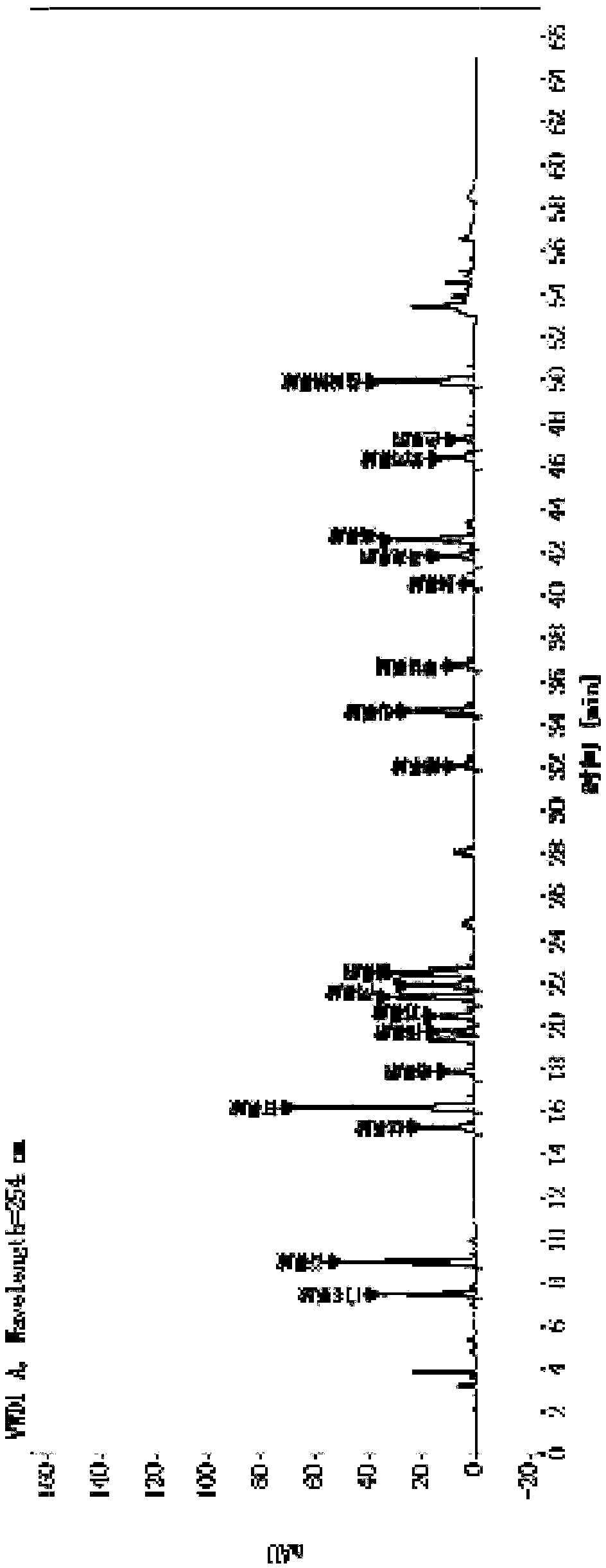
[0085] Example 2 Determination of polypeptide content in transfer factor capsules by "amino acid difference method"
[0086] 1. Chromatographic conditions
[0087] Adopt Agilent high-performance liquid chromatography, use octadecylsilane bonded silica gel as filler chromatographic column; use methanol-acetonitrile-water (20:60:20) as mobile phase A, and use 0.1mol / L sodium acetate buffer solution (Adjust the pH value to 6.3 with glacial acetic acid) for gradient elution with mobile phase B; the flow rate is 1.0ml per minute, the detection wavelength is 254nm, the column temperature is 35°C, and the injection volume is 2μl.
[0088] Table 5 Elution Gradient Schedule
[0089]
[0090]
[0091] 2. Determination of standard curve:
[0092] Preparation of reference substance stock solution Accurately weigh 15.0 mg of glutamic acid reference substance, aspartic acid, serine, glycine, histidine, arginine, threonine, alanine, proline, tyrosine, Valine, methionine, cystine, is...
PUM
| Property | Measurement | Unit |
|---|---|---|
| mobile phase | aaaaa | aaaaa |
| length | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com