HPLC measurement method for content of multi-index components in rhizoma corydalis pain relieving drop pills
A technology of Yuanhu Zhitong dripping pills and its determination method, which is applied in the field of content determination of Chinese patent medicines, can solve the problems that it is difficult to fully reflect the quality of Yuanhu Zhitong dripping pills, restrict market expansion, and the internationalization of clinical applications, and achieve the reduction of testing costs and Time, accurate control, taking safe and effective effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
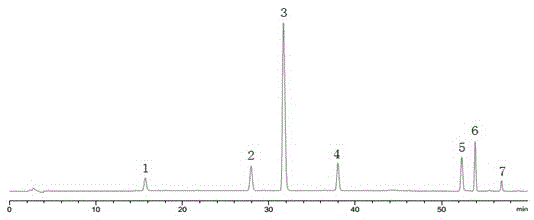
[0046] 3.1 Preparation of mixed reference solution
[0047] Take an appropriate amount of reference substances of proto-opiateline, palmatine, dehydrocordate, corydaline, imperatorin, imperatorin and isoimperatorin, weigh them accurately, and add methanol solution to make each mL of A mixed solution of 20 μg of opium, 10 μg of palmatine, 80 μg of dehydrocordate, 40 μg of corydalidin, 40 μg of corydalidin, 20 μg of imperatorin, and 10 μg of isoimperatorin, and shake well to obtain the product.
[0048] 3.2 Preparation of the test solution
[0049] Take Yuanhu Zhitong Dropping Pills and grind them finely, take 1g of Yuanhu Zhitong Dropping Pills fine powder, weigh them accurately, put them in a stoppered conical flask, add 50ml of methanol solution, and weigh them; ultrasonically treat for 30min, let cool, and weigh Determine the weight, make up the lost weight with methanol solution, shake well, filter; accurately measure 25ml of the filtrate, steam to nearly dry, dissolve the...
Embodiment 1
[0090] A kind of HPLC assay method of multi-index component content in Yuanhu Zhitong dripping pill, the method comprises the following steps:
[0091] (1) Chromatographic conditions: the chromatographic column is DiamonsilC 18 Column (250mm×4.6mm, 5μm), the chromatographic column is filled with octadecylsilane bonded silica gel; detection wavelength: 280nm; mobile phase: phase A is acetonitrile, phase B is glacial acetic acid solution with a volume concentration of 0.2%, Triethylamine was used to adjust the pH of glacial acetic acid solution to 5.0; using gradient elution, the volume ratio of acetonitrile to mobile phase was: 0min, 20%; 10min, 22%; 30min, 30%; 60min, 80%; 65min, 100%; Flow rate: 1.0 mL / min; column temperature: 35°C.
[0092] (2) Preparation of mixed reference substance solution: Take appropriate amount of reference substances of protopine, patellae, dehydrotetrahydrotetrahydropalmatine, corydaline, imperatorin and isoimperatorin, and weigh them precisely. ,...
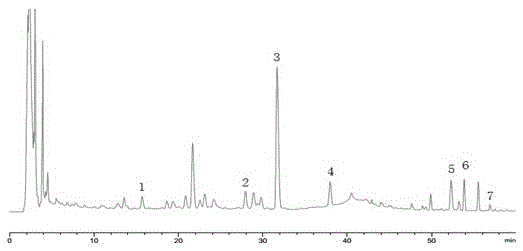
Embodiment 2
[0101] A kind of HPLC assay method of multi-index component content in Yuanhu Zhitong dripping pill, the method comprises the following steps:
[0102] (1) Chromatographic conditions: the chromatographic column is DiamonsilC 18 Column (250mm×4.6mm, 5μm), the chromatographic column is filled with octadecylsilane bonded silica gel; detection wavelength: 300nm; mobile phase: phase A is acetonitrile, phase B is glacial acetic acid solution with a volume concentration of 1.0%, Triethylamine was used to adjust the pH of glacial acetic acid solution to 5.0; using gradient elution, the volume ratio of acetonitrile to mobile phase was: 0min, 20%; 10min, 22%; 30min, 30%; 60min, 80%; 65min, 100%; Flow rate: 1.0 mL / min; column temperature: 40°C.
[0103] (2) Preparation of mixed reference substance solution: Take appropriate amount of reference substances of protopine, patellae, dehydrotetrahydrotetrahydropalmatine, corydaline, imperatorin and isoimperatorin, and weigh them precisely. ,...
PUM
| Property | Measurement | Unit |
|---|---|---|
| wavelength | aaaaa | aaaaa |
| recovery rate | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com