Clevidipine butyrate injection
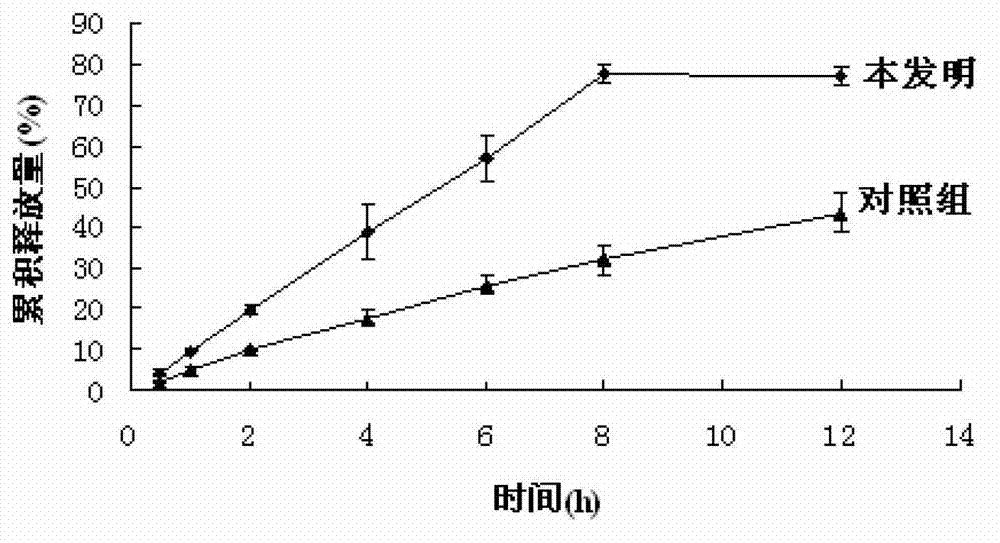
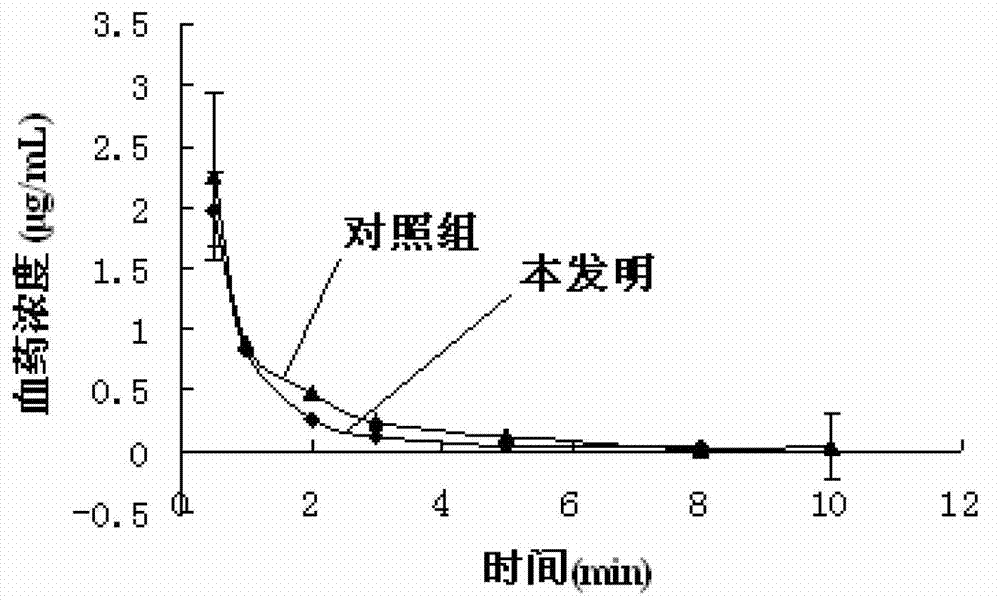
A technology of clevidipine butyrate and dipine injection, which is applied in the fields of emulsion delivery, cardiovascular system diseases, organic active ingredients, etc., can solve the problems of easy aggregation and merging of emulsion droplets, thermodynamic instability, demulsification and floating oil, etc., and achieves Reduce the lipid peroxidation problem in the body, prolong the storage time, and reduce the oil phase content
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0043] The clevidipine butyrate injection provided in this embodiment includes clevidipine butyrate, oil phase, phospholipid and organic solvent, and the weight percentages of each component are as follows:
[0044] Clevidipine butyrate 0.01-10%,
[0045] Oil phase 0.1-10%,
[0046] Phospholipids 5-50%,
[0047] Organic solvent 40-90%.
[0048] In the above prescription, the oil phase includes at least one of the following: soybean oil, corn oil, castor oil, olive oil, coconut oil, peanut oil, fish oil, cottonseed oil, glyceryl monostearate, glyceryl monooleate, sesame oil , safflower oil, C 6 -C 8 Fatty acid triglycerides, ethyl oleate.
[0049] The phospholipids include at least the following: natural phospholipids and synthetic phospholipids.
[0050] The organic solvent includes at least the following: ethanol, glycerol, propylene glycol, polyethylene glycol, wherein ethanol is preferred.
[0051] The preparation method of above-mentioned injection comprises the fol...
Embodiment 2
[0073] prescription:
[0074]
[0075] Preparation:
[0076] Accurately weigh the prescribed quantity of clevidipine butyrate, dissolve it in part of absolute ethanol, then add lecithin E-80, soybean oil and the remaining prescribed amount of absolute alcohol, stir to dissolve each component and mix well Clevidipine Acid Injection.
[0077] In clinical application, the above prescription is diluted 5-100 times (0.1-2 mg / mL) for better effect, more preferably 0.5 mg / mL.
Embodiment 3
[0080] prescription:
[0081]
PUM
| Property | Measurement | Unit |
|---|---|---|
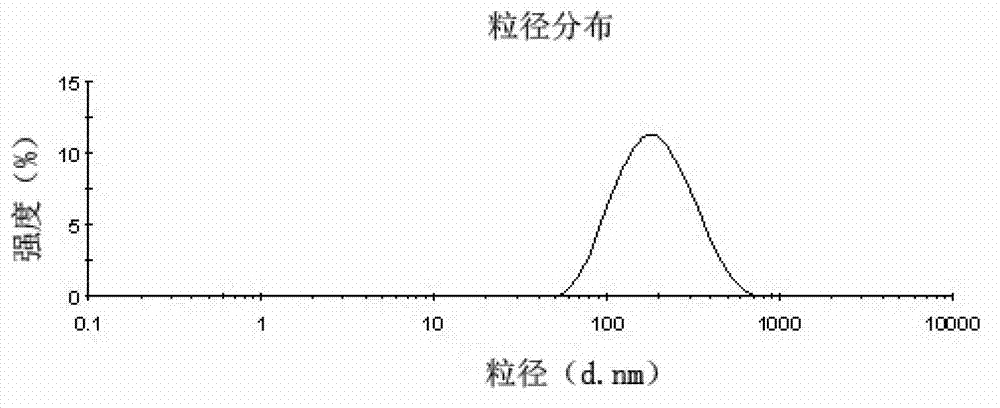
| The average particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com