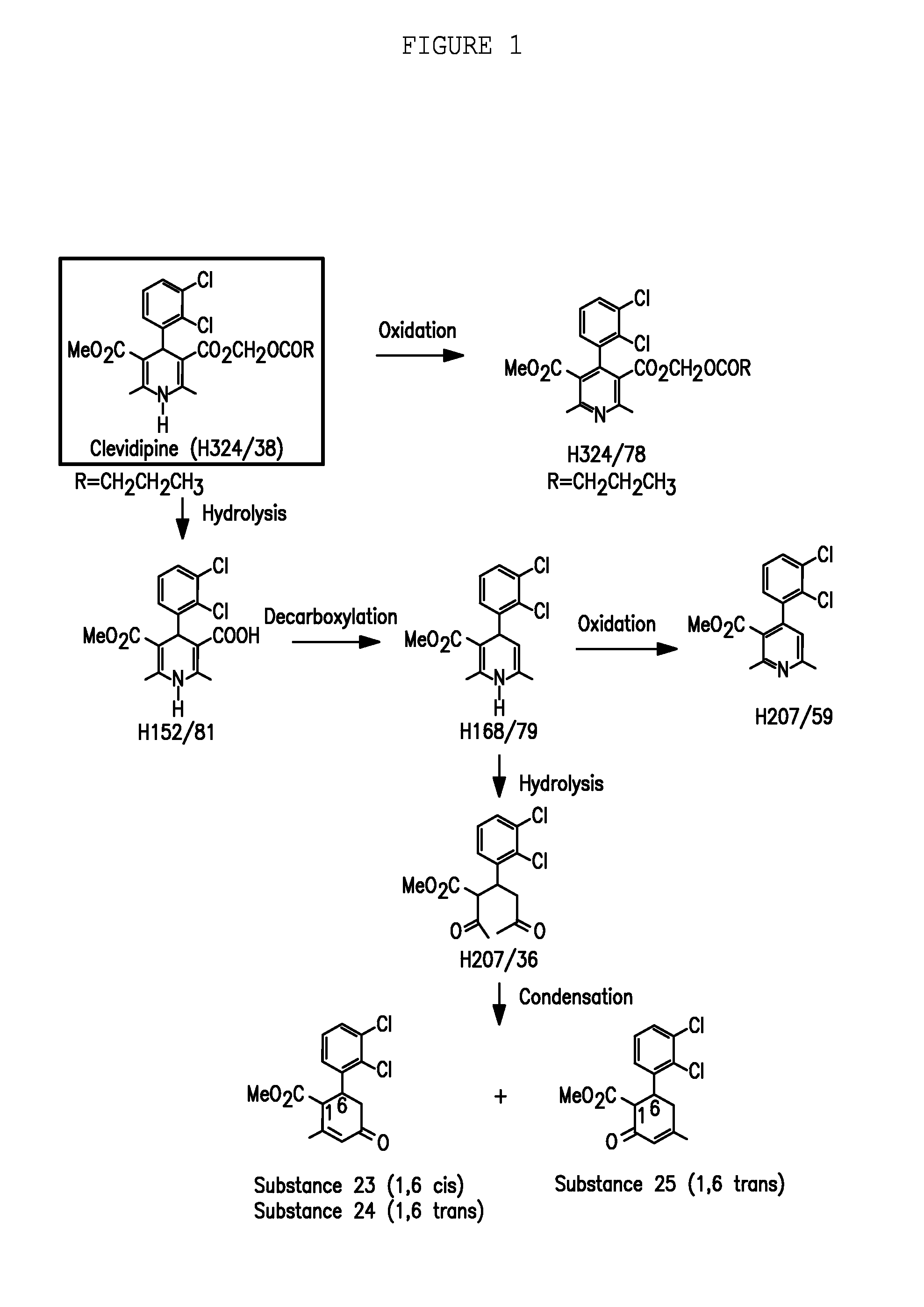
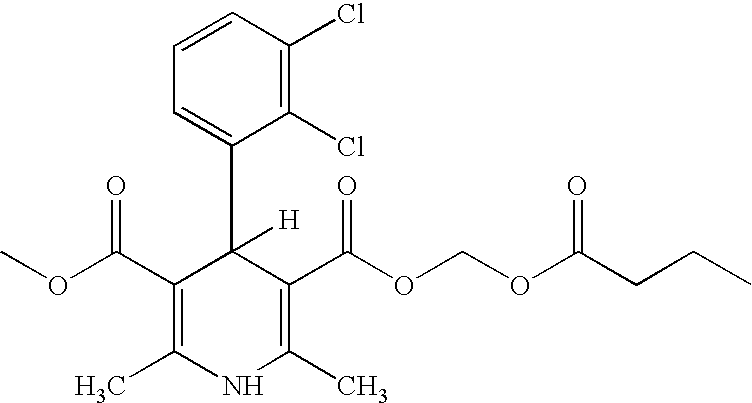
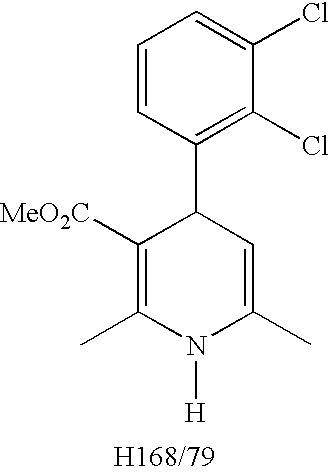
Pharmaceutical Compositions And Methods For Producing Low Impurity Concentrations Of The Same
a technology of compositions and pharmaceutical compositions, applied in the field of pharmaceutical compositions, to achieve the effect of reducing or minimizing the level of certain impurities
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
HPLC Procedure
[0035]Clevidipine assay and related substances were tested at each time point by a stability indicating method. This method is an isocratic, normal phase HPLC method with peak detection at 220 nm wavelength.
Column temperature: 35-40 degrees C.
Injection volume: 20 μl.
Flow rate: 1.0 ml / min.
Run time about 25 minutes.
Mobile phase of Heptane:ethanol (90:10) is employed and used for the assay of clevidipine and the degradation products with the exception of Substance 24.
Condition column with clevidipine mobile phase at 1.0 mL / min for 4 hours.
New column should be conditioned overnight at 0.2 mL / min.
When a degradation product is eluted, column can be washed with filtered ethanol for about 2 hours at 1.0 mL / min, then proceed with equilibration.
Examples of Column: PVA silica column 4.6 mm×150 mm, 5 micron PV12s051546WT or equivalent.
example 2
HPLC Procedure Substance 24
[0036]This method is an isocratic, normal phase HPLC method with peak detection at 220 nm wavelength.
Column temperature: 35-40 degrees C.
Injection volume: 20 μl to 100 μl.
Run time about 60 minutes.
Mobile phase of Heptane:Isopropyl Alcohol (95:5) is employed is used for the assay of Substance 24.
Condition column with Heptane:Isopropyl Alcohol 95:5 mobile phase at 1.0 ml / min until the blank injection baseline is stable. New column should be conditioned overnight at 0.2 mL / min.
Examples of Column: Two PVA silica columns 4.6 mm×150 mm, 5 micron PV12s051546WT or equivalent.
Flow rate 1.0 mL / min.
[0037]Calculation of percent impurity based on total peak area:
impurityPeakArea(100)(totalpeakareaofdegradationproducts+H324 / 38peakarea(clevidipinepeakarea))
[0038]Calculation of percent impurity based on total peak area using H168 / 79 as the impurity example:
H168 / 79PeakArea(100)(totalpeakareaofdegradationproducts+H324 / 38peakarea(clevidipinepeakarea))
[0039]When a standard of...
PUM
| Property | Measurement | Unit |
|---|---|---|
| temperature | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
| temperatures | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com