Clevidipine butyrate liquid liposome preparation
A technology of clevidipine butyrate and liquid lipids, which can be used in liposome delivery, medical preparations containing non-active ingredients, medical preparations containing active ingredients, etc., and can solve problems such as potential safety hazards
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
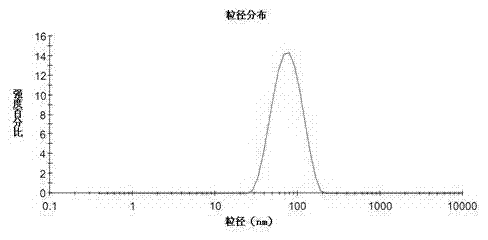
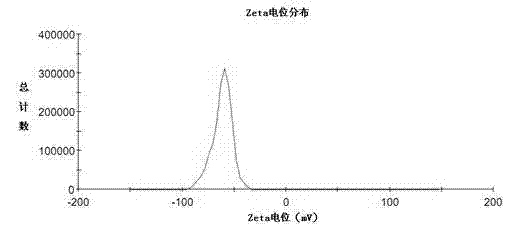
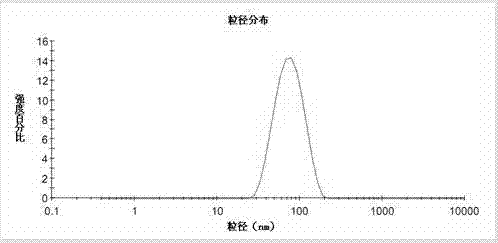
Image
Examples
Embodiment 1
[0028] prescription:
[0029] Clevidipine Butyrate 1g
[0030] Soy Phosphatidylcholine 200g
[0031] Soy Phosphatidylglycerol 80g
[0032] Cholesterol 50g
[0033] Oleic acid 2g
[0034] Vitamin E 0.05g
[0035]
[0036] Make 1000ml
[0037] Preparation Process:
[0038] (1) Stir and dissolve clevidipine butyrate in 100ml of chloroform:methanol (1:1, volume ratio) mixed solution.
[0039] (2) Dissolve soybean phosphatidylglycerol in an appropriate amount of chloroform:methanol (1:1, volume ratio) mixed solution completely, and then mix with (1) solution.
[0040] (3) Dissolve soybean phosphatidylcholine and cholesterol in 100ml of chloroform:methanol (1:1, volume ratio) mixed solution, then slowly drop in oleic acid and vitamin E to dissolve completely.
[0041] (4) After mixing the solution obtained in (2) and the solution obtained in (3) evenly, use a spray dryer to remove the organic solvent to obt...
Embodiment 2
[0046] prescription:
[0047]Clevidipine Butyrate 1g
[0048] Soy Phosphatidylcholine 200g
[0049] Soy Phosphatidylglycerol 80g
[0050] Cholesterol 50g
[0051] Vitamin E 0.05g
[0052]
[0053] Make 1000ml
[0054] Preparation Process:
[0055] (1) Stir and dissolve clevidipine butyrate in 100ml of chloroform:methanol (1:1, volume ratio) mixed solution.
[0056] (2) Dissolve soybean phosphatidylglycerol in an appropriate amount of chloroform:methanol (1:1, volume ratio) mixed solution completely, and then mix with (1) solution.
[0057] (3) Dissolve soybean phosphatidylcholine and cholesterol in 100ml of chloroform:methanol (1:1, volume ratio) mixed solution, and then slowly drop vitamin E to dissolve it completely.
[0058] (4) After mixing the solution obtained in (2) and the solution obtained in (3) evenly, use a spray dryer to remove the organic solvent to obtain a spray-dried powder.
[0059] (5) Take a certain amount o...
Embodiment 3
[0063] prescription:
[0064] Clevidipine Butyrate 1g
[0065] Egg Yolk Phosphatidylcholine 230g
[0066] Egg Yolk Phosphatidylglycerol 70 g
[0067] Stigmasterol 70g
[0068] Sodium Oleate 4g
[0069] Coenzyme Q10 0.1g
[0070]
[0071] Make 1000ml
[0072] Preparation Process:
[0073] (1) Stir and dissolve clevidipine butyrate in 100ml of chloroform:methanol (1:1, volume ratio) mixed solution.
[0074] (2) Dissolve egg yolk phosphatidylglycerol in an appropriate amount of chloroform:methanol (1:1, volume ratio) mixed solution completely, and then mix with (1) solution.
[0075] (3) Dissolve egg yolk phosphatidylcholine and stigmasterol in 100ml of chloroform:methanol (1:1, volume ratio) mixed solution, then slowly drop in oleic acid and coenzyme Q10 to dissolve completely.
[0076] (4) After mixing the solution obtained in (2) and the solution obtained in (3) evenly, use a spray dryer to remove the organic solv...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com