Clevidipine butyrate intralipid and preparation method thereof
A technology of clevidipine butyrate and fat emulsion, which is applied in the directions of oil/fat/wax inactive ingredients, pharmaceutical formulations, emulsion delivery, etc. Thermodynamic instability and other problems, to achieve the effects of low preparation cost, low biological toxicity, and improved physical stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
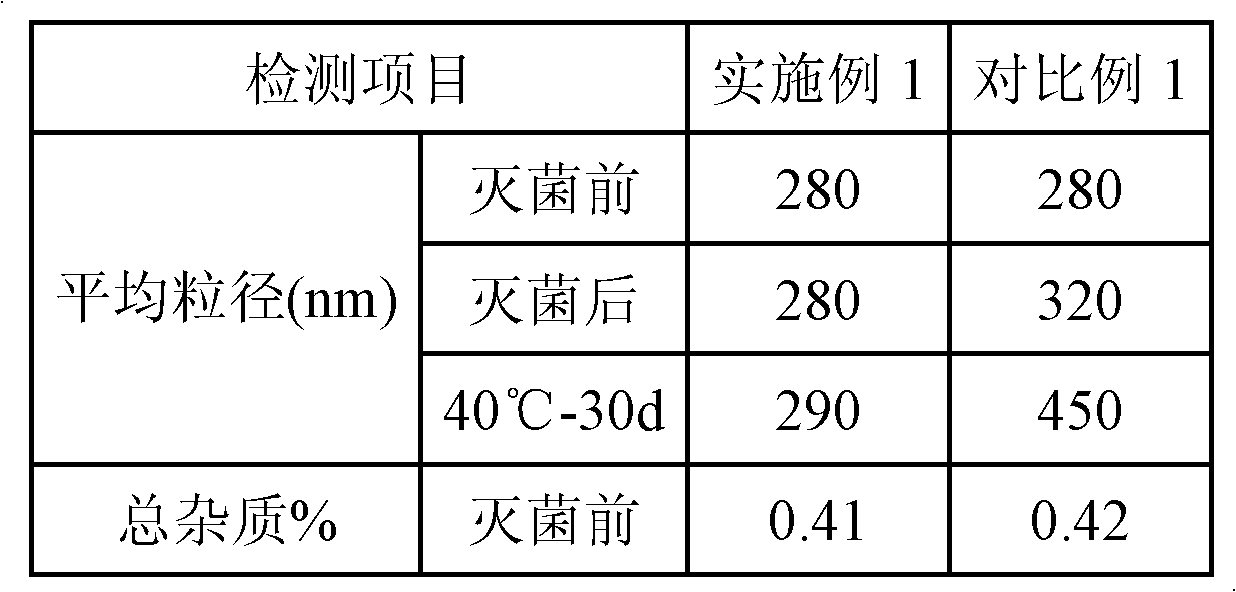
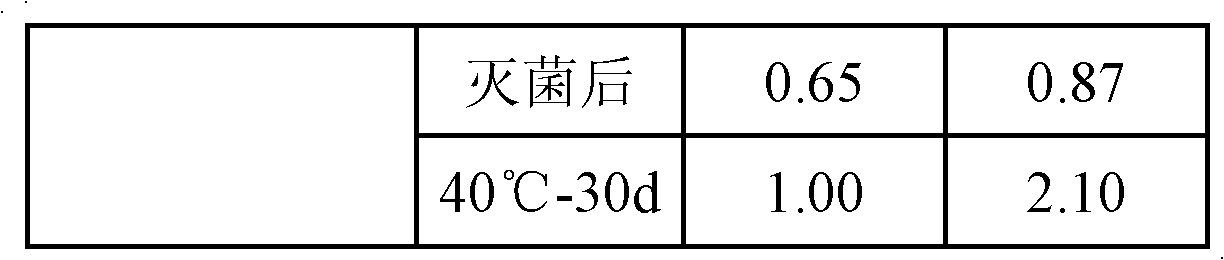
Embodiment 1
[0033] a) Prepare the oil phase: dissolve and disperse 0.05g of clevidipine butyrate active ingredient, 1.2g of lecithin, 0.1g of distearoylphosphatidylglycerol (DSPG), and 0.1g of vitamin E in 20g of medium-chain triglycerides (MCT ), heated to 60°C for use;
[0034] b) Preparation of water phase: Dissolve and disperse 2.3g glycerin and 0.005g EDTA-2Na in 60mL water for injection, heat to 60°C for use;
[0035] c) mixing and shearing the prepared oil phase and water phase to form colostrum;
[0036] d) dilute the volume of colostrum to 100mL with water for injection, and then circulate and homogenize 6 times under a pressure of 800bar to obtain end milk;
[0037] e) adding NaOH to adjust pH=8.0;
[0038] f) After filtering with a microporous membrane with a pore size of 0.8 μm, fill with nitrogen, and sterilize to obtain the clevidipine butyrate fat emulsion.
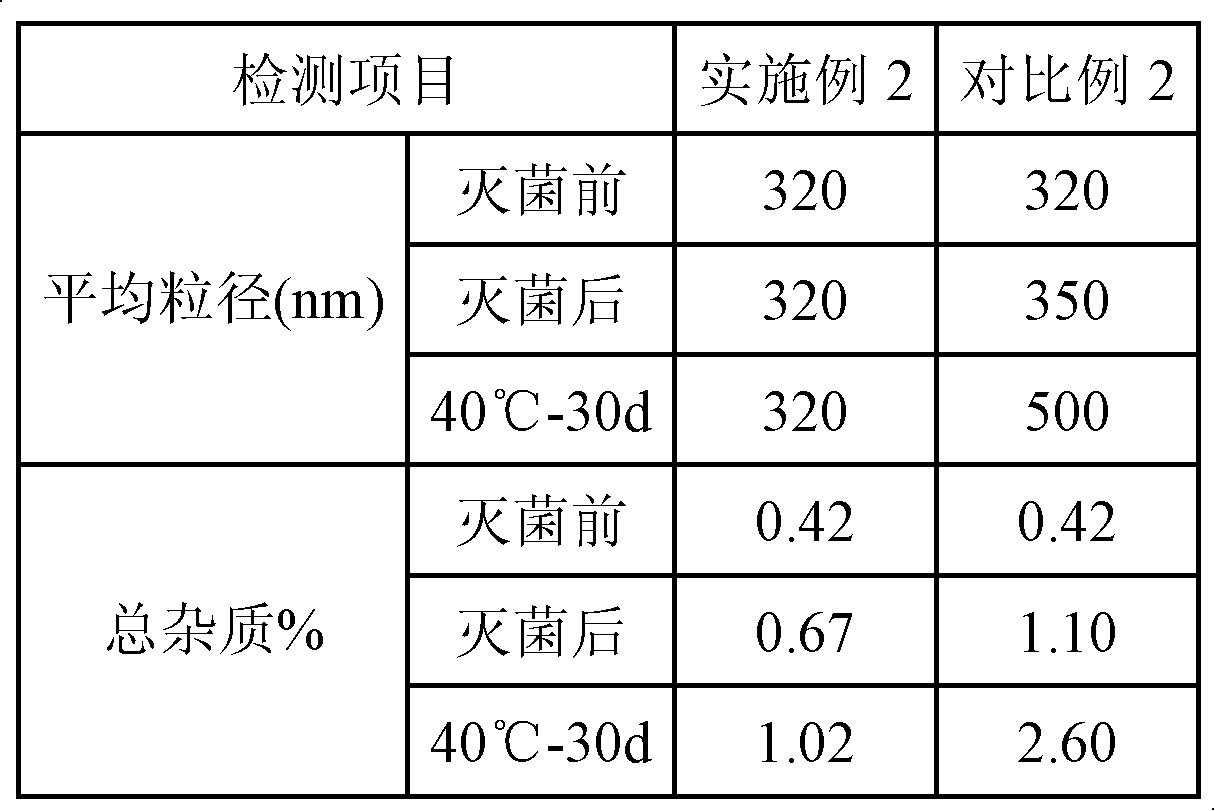
Embodiment 2
[0046] a) Prepare the oil phase: Dissolve and disperse 0.05g clevidipine butyrate active ingredient, 1.2g lecithin, 0.5g distearoylphosphatidylglycerol (DSPG) in 20g medium chain triglycerides (MCT), heat to 80°C for use;
[0047] b) Prepare the water phase: dissolve and disperse 2.3g of glycerin in 60mL of water for injection, heat to 80°C for use;
[0048] c) mixing and shearing the prepared oil phase and water phase to form colostrum;
[0049] d) dilute the volume of colostrum to 100mL with water for injection, and then circulate and homogenize 8 times under a pressure of 600bar to obtain end milk;
[0050] e) adding NaOH to adjust pH=8.0;
[0051] f) After filtering with a microporous membrane with a pore size of 0.8 μm, fill with nitrogen, and sterilize to obtain the clevidipine butyrate fat emulsion.
Embodiment 3
[0058] a) Prepare the oil phase: dissolve and disperse 0.05g of clevidipine butyrate active ingredient, 1.2g of lecithin, and 1.0g of distearoylphosphatidylglycerol (DSPG) in 20g of medium-chain triglycerides (MCT), and heat to 70°C for use;
[0059] b) Prepare the water phase: dissolve and disperse 2.3g of glycerin in 60mL of water for injection, heat to 70°C for use;
[0060] c) mixing and shearing the prepared oil phase and water phase to form colostrum;
[0061] d) dilute the volume of colostrum to 100mL with water for injection, and then circulate and homogenize it 4 times under a pressure of 1000bar to obtain end milk;
[0062] e) adding NaOH to adjust pH=8.0;
[0063] f) After filtering with a microporous membrane with a pore size of 0.8 μm, fill with nitrogen, and sterilize to obtain the clevidipine butyrate fat emulsion.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com