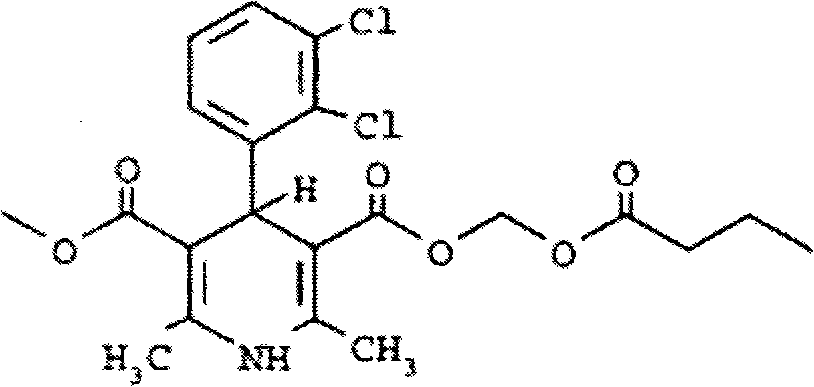
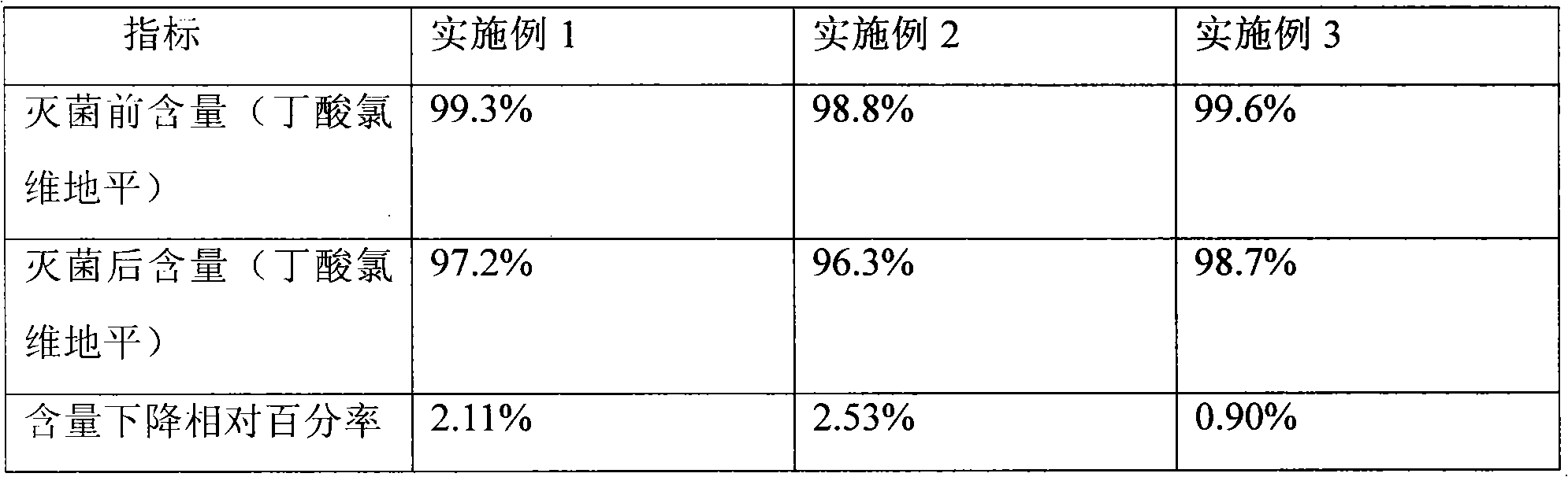
Clevidipine butyrate medium-length chain fat emulsion and preparation method thereof
A technology of clevidipine butyrate and medium-long chain, which is applied in the direction of non-active ingredients of oil/fat/wax, pharmaceutical formula, emulsion delivery, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] prescription
[0030] Clevidipine Butyrate 0.5g
[0031] Refined soybean oil 200g
[0033] Glycerin 22.5g
[0034] Appropriate amount of sodium hydroxide
[0035]
[0036] Add water for injection to 1000ml
[0037] Process:
[0038] (1) Preparation of water phase: add glycerin to water to dissolve, heat to 75°C, and set aside;
[0039] (2) Preparation of oil phase: heat refined soybean oil to 75°C, add egg yolk lecithin (with a phosphatidylcholine content of more than 80%) to dissolve, add clevidipine butyrate, and stir to dissolve;
[0040] (3) Preparation of colostrum: Add the oil phase of step (2) into the water phase of step (1), at a temperature of 75° C., high-speed shear dispersion, shear speed of 8000 rpm, and time of 15 minutes to form colostrum;
[0041] (4) Adjusting the pH value: rapidly cooling the colostrum in step (3) below 30°C, and adjusting the pH value to 6.0-8.0 with sodium hydr...
Embodiment 2
[0046] prescription
[0047] Clevidipine Butyrate 0.5g
[0048] Medium Chain Triglycerides 200g
[0050] Glycerin 22.5g
[0051] Appropriate amount of sodium hydroxide
[0052]
[0053] Add water for injection to 1000ml
[0054] Process:
[0055] (1) Preparation of water phase: add glycerin to water to dissolve, heat to 75°C, and set aside;
[0056] (2) Preparation of oil phase: heat refined soybean oil to 75°C, add egg yolk lecithin (with a phosphatidylcholine content of more than 80%) to dissolve, add clevidipine butyrate, and stir to dissolve;
[0057] (3) Preparation of colostrum: Add the oil phase of step (2) into the water phase of step (1), at a temperature of 75° C., high-speed shear dispersion, shear speed of 8000 rpm, and time of 15 minutes to form colostrum;
[0058] (4) pH value adjustment: quickly cool the colostrum in step (3) below 30°C, and adjust the pH value to 6.0~ with sodium hydroxide
...
Embodiment 3
[0063] prescription:
[0064] Clevidipine Butyrate 0.5g
[0065] Refined soybean oil 100g
[0066] Medium Chain Triglycerides 100g
[0067] Egg yolk lecithin 12g
[0068] Glycerin 22.5g
[0069] Appropriate amount of sodium hydroxide
[0070]
[0071] Add water for injection to 1000ml
[0072] Process:
[0073] (1) Preparation of water phase: add glycerin to water to dissolve, heat to 75°C, and set aside;
[0074] (2) Preparation of oil phase: heat refined soybean oil and medium-chain triglycerides to 75°C, add egg yolk lecithin (more than 80% phosphatidylcholine content) to dissolve, add clevidipine butyrate, and stir to make it dissolve;
[0075] (3) Preparation of colostrum: Add the oil phase of step (2) into the water phase of step (1), at a temperature of 75° C., high-speed shear dispersion, shear speed of 8000 rpm, and time of 15 minutes to form colostrum;
[0076] (4) pH value adjustment: quickly cool down the colostrum in step (...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com