Method for detecting clevidipine butyrate and related substances in preparations of clevidipine butyrate
A clevidipine butyrate and detection method technology, applied in the field of medicine, can solve problems such as interference, inability to separate, and no uniform drug standards for drugs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
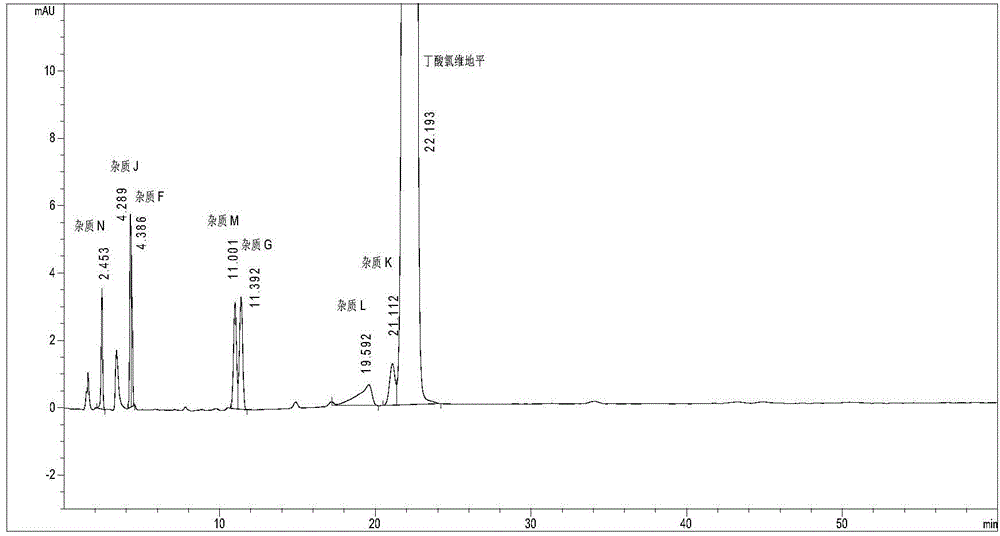
[0049] Embodiment 1 acetonitrile: methanol: phosphate buffer solution (pH=3.0)=40:20:40 (volume ratio) is the test that the mobile phase cannot completely separate the mixed solution of known impurities
[0050] 1. Instruments: Agilent 1260 high performance liquid chromatography, electronic analytical balance, pH meter.
[0051] 2. Determination method: Accurately weigh an appropriate amount of reference substances of clevidipine butyrate, impurity G, impurity F and reference substances of H324 / 78, H168 / 79, H207 / 59, H152 / 81 and H152 / 66, add methanol to dissolve And be diluted into the known impurity mixed solution that contains clevidipine butyrate 1mg and each impurity about 1 μ g in every 1ml as need testing solution. Chromatographic column Waters sunfire C18 150×4.6mm, 3.5μm; mobile phase is acetonitrile: methanol: phosphate buffer solution = 40:20:40, phosphate buffer solution consists of 15ml 1mol / L phosphoric acid and 100ml 1mol / L sodium dihydrogen phosphate Mix, add wa...
Embodiment 2
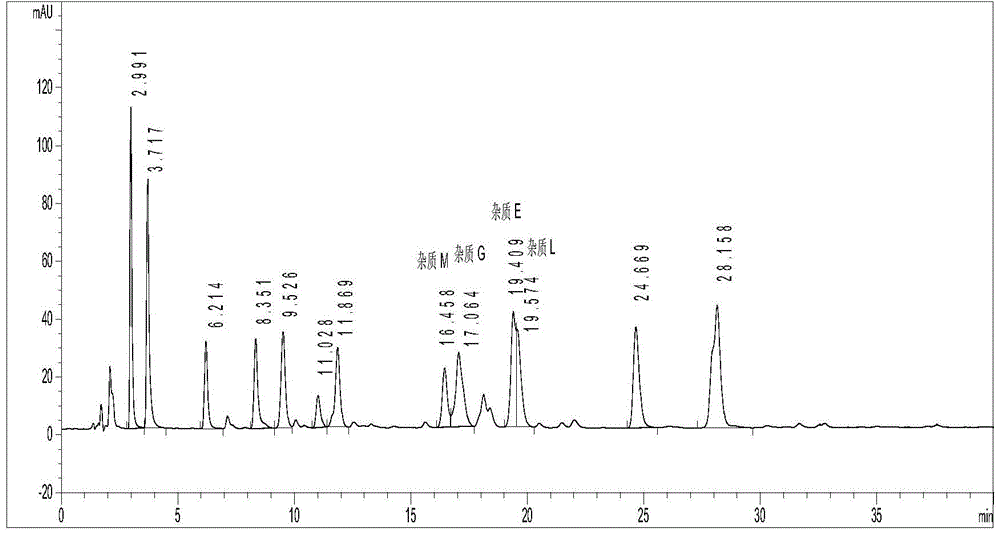
[0052] Embodiment 2 acetonitrile: methanol: phosphate buffer solution (pH=3.0)=40:20:40 (volume ratio) is the test that the mobile phase cannot completely separate the mixed solution of known impurities
[0053] 1. Instruments: Agilent 1260 high performance liquid chromatography, electronic analytical balance, pH meter.
[0054] 2. Determination method (1): Accurately weigh an appropriate amount of reference substances of impurities A~I and reference substances of H324 / 78, H168 / 79, H207 / 59, H152 / 81 and H152 / 66, add methanol to dissolve and dilute to each A mixed solution of known impurities containing about 20 μg of each impurity in 1 ml was used as the test solution. Chromatographic column Hypersil BDS C18150×4.6mm, 3μm; mobile phase is acetonitrile:methanol:phosphate buffer solution=40:20:40, phosphate buffer solution consists of 15ml 1mol / L phosphoric acid and 100ml 1mol / L phosphoric acid di Mix sodium hydrogen, add water to dilute to 2000ml, and adjust pH to 3.0 with phos...
Embodiment 3
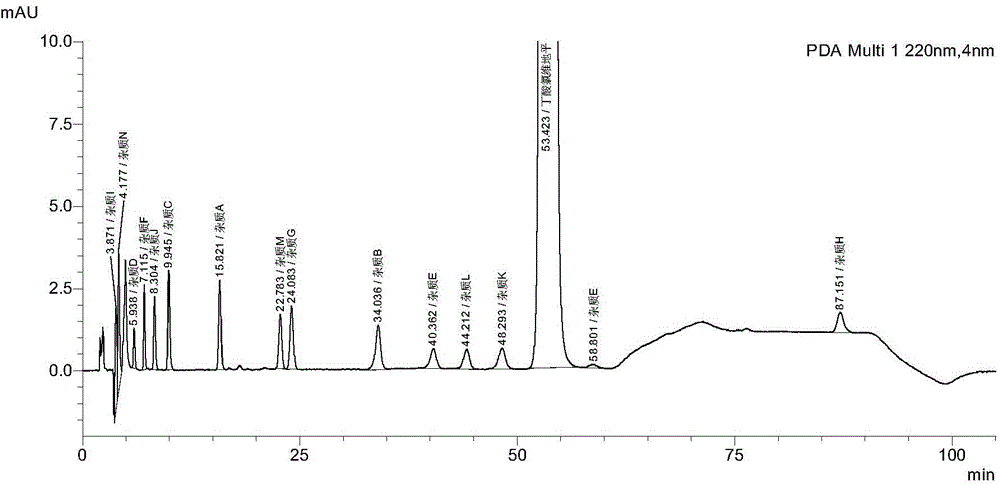
[0055] Example 3 Acetonitrile-0.01mol / L ammonium dihydrogen phosphate buffer solution as the mobile phase and gradient elution with different ratios can not completely separate the test of the mixed solution of known impurities
[0056] 1. Instruments: Agilent 1260 high performance liquid chromatography, electronic analytical balance, pH meter.
[0057] 2. Determination method (1): Accurately weigh the reference substances of clevidipine butyrate, impurity G, impurity F and the reference substances of H324 / 78, H168 / 79, H207 / 59, H152 / 81 and H152 / 66. Add methanol to dissolve and dilute to a mixed solution of known impurities containing 1 mg clevidipine butyrate and about 1 μg of each impurity in every 1 ml as the test solution. Chromatographic column Waters sunfire C18 150×4.6mm, 3.5μm; mobile phase system, A is acetonitrile, B is 0.01mol / L ammonium dihydrogen phosphate buffered saline buffer solution, 0.01mol / L ammonium dihydrogen phosphate buffer solution is prepared by 1.15g ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
| Column length | aaaaa | aaaaa |
| Column inner diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com